Page 102 - Gas Purification 5E
P. 102
92 GasPurifcation
210 230 250 270 290 310 330 350 370
BOILING POINT, 'F.
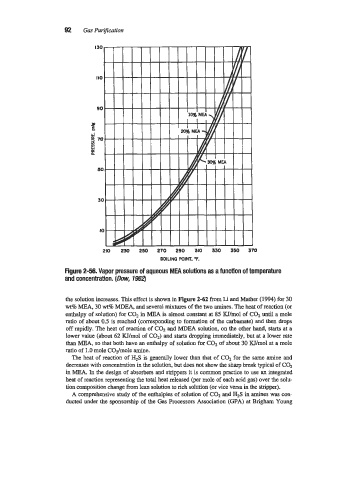
Figure 2-58. Vapor pressure of aqueous MEA solutions as a function of temperature
and concentration. (Dow, 7962)
the solution increases. This effect is shown in Figure 2-62 from Li and Mather (1994) for 30
wt% MEA, 30 wt% MDEA, and several mixtures of the two amines. The heat of rcaction (or
enthalpy of solution) for C02 in MEA is almost constant at 85 KJlmol of C02 until a mole
ratio of about 0.5 is reached (corresponding to formation of the carbamate) and then drops
off rapidly. The heat of reaction of C02 and MDEA solution, on the other hand, starts at a
lower value (about 62 KJlmol of Cod and starts dropping immediately, but at a lower rate
than MEA, so that both have an enthalpy of solution for CO, of about 30 Who1 at a mole
ratio of 1 .O mole C02/mole amine.
The heat of reaction of H2S is generally lower than that of CO, for the same amine and
decreases with concentration in the solution, but does not show the sharp break typical of CQ
in MEA. In the design of absorbers and strippem it is common practice to use an integ.ltea
heat of don representing the total heat released @er mole of each acid gas) over the sole
tion composition change from lean solution to rich solution (or vice versa in the stripper).
A comprehensive study of the enthalpies of solution of CO, and H2S in amines was con-
ducted under the sponsorship of the Gas Processors Association (GPA) at Brigham Young