Page 111 - Gas Wettability of Reservoir Rock Surfaces with Porous Media
P. 111
Gas-wetting Alteration Agent and Gas-wetting CHAPTER 3 95
G04-AA-DA
G04-AA
4000 3500 3000 2500 2000 1500 1000 500
Wave number/cm –1
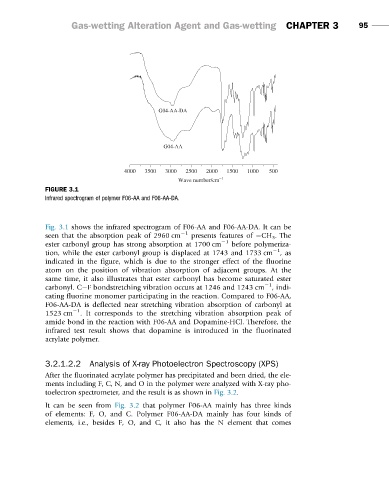
FIGURE 3.1
Infrared spectrogram of polymer F06-AA and F06-AA-DA.
Fig. 3.1 shows the infrared spectrogram of F06-AA and F06-AA-DA. It can be
seen that the absorption peak of 2960 cm 21 presents features of CH 3 . The
ester carbonyl group has strong absorption at 1700 cm 21 before polymeriza-
tion, while the ester carbonyl group is displaced at 1743 and 1733 cm 21 ,as
indicated in the figure, which is due to the stronger effect of the fluorine
atom on the position of vibration absorption of adjacent groups. At the
same time, it also illustrates that ester carbonyl has become saturated ester
carbonyl. C F bondstretching vibration occurs at 1246 and 1243 cm 21 ,indi-
cating fluorine monomer participating in the reaction. Compared to F06-AA,
F06-AA-DA is deflected near stretching vibration absorption of carbonyl at
1523 cm 21 . It corresponds to the stretching vibration absorption peak of
amide bond in the reaction with F06-AA and Dopamine-HCl. Therefore, the
infrared test result shows that dopamine is introduced in the fluorinated
acrylate polymer.
3.2.1.2.2 Analysis of X-ray Photoelectron Spectroscopy (XPS)
After the fluorinated acrylate polymer has precipitated and been dried, the ele-
ments including F, C, N, and O in the polymer were analyzed with X-ray pho-
toelectron spectrometer, and the result is as shown in Fig. 3.2.
It canbeseenfrom Fig. 3.2 that polymer F06-AA mainly has three kinds
of elements: F, O, and C. Polymer F06-AA-DA mainly has four kinds of
elements, i.e., besides F, O, and C, it also has the N element that comes