Page 111 - Gas Adsorption Equilibria
P. 111
2. Volumetry / Manometry 97
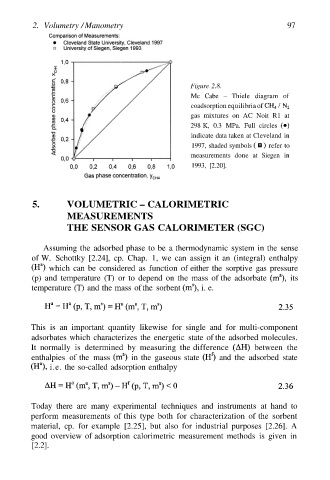
Figure 2.8.
Mc Cabe – Thiele diagram of
coadsorption equilibria of
gas mixtures on AC Noit R1 at
298 K, 0.3 MPa. Full circles
indicate data taken at Cleveland in
1997, shaded symbols refer to
measurements done at Siegen in
1993, [2.20].
5. VOLUMETRIC – CALORIMETRIC
MEASUREMENTS
THE SENSOR GAS CALORIMETER (SGC)
Assuming the adsorbed phase to be a thermodynamic system in the sense
of W. Schottky [2.24], cp. Chap. 1, we can assign it an (integral) enthalpy
which can be considered as function of either the sorptive gas pressure
(p) and temperature (T) or to depend on the mass of the adsorbate its
temperature (T) and the mass of the sorbent i. e.
This is an important quantity likewise for single and for multi-component
adsorbates which characterizes the energetic state of the adsorbed molecules.
It normally is determined by measuring the difference between the
enthalpies of the mass in the gaseous state and the adsorbed state
i.e. the so-called adsorption enthalpy
Today there are many experimental techniques and instruments at hand to
perform measurements of this type both for characterization of the sorbent
material, cp. for example [2.25], but also for industrial purposes [2.26]. A
good overview of adsorption calorimetric measurement methods is given in
[2.2].