Page 227 - Gas Adsorption Equilibria
P. 227
4. Volumetric – Gravimetric Measurements 213
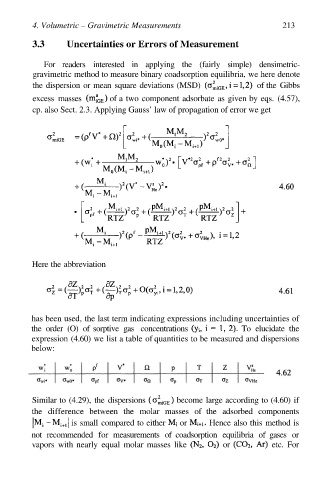
3.3 Uncertainties or Errors of Measurement
For readers interested in applying the (fairly simple) densimetric-
gravimetric method to measure binary coadsorption equilibria, we here denote
the dispersion or mean square deviations (MSD) of the Gibbs
excess masses of a two component adsorbate as given by eqs. (4.57),
cp. also Sect. 2.3. Applying Gauss’ law of propagation of error we get
Here the abbreviation
has been used, the last term indicating expressions including uncertainties of
the order (O) of sorptive gas concentrations To elucidate the
expression (4.60) we list a table of quantities to be measured and dispersions
below:
Similar to (4.29), the dispersions become large according to (4.60) if
the difference between the molar masses of the adsorbed components
is small compared to either or Hence also this method is
not recommended for measurements of coadsorption equilibria of gases or
vapors with nearly equal molar masses like or etc. For