Page 308 - Gas Adsorption Equilibria
P. 308
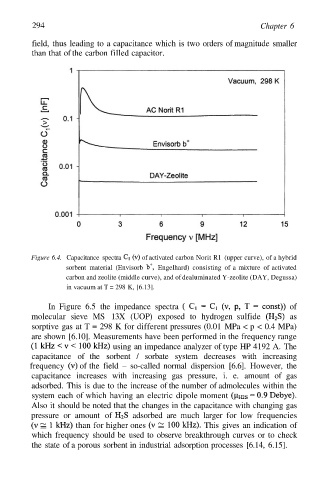
294 Chapter 6
field, thus leading to a capacitance which is two orders of magnitude smaller
than that of the carbon filled capacitor.
Figure 6.4. Capacitance spectra of activated carbon Norit R1 (upper curve), of a hybrid
sorbent material (Envisorb Engelhard) consisting of a mixture of activated
carbon and zeolite (middle curve), and of dealuminated Y-zeolite (DAY, Degussa)
in vacuum at T = 298 K, [6.13].
In Figure 6.5 the impedance spectra of
molecular sieve MS 13X (UOP) exposed to hydrogen sulfide as
sorptive gas at T = 298 K for different pressures (0.01 MPa < p < 0.4 MPa)
are shown [6.10]. Measurements have been performed in the frequency range
using an impedance analyzer of type HP 4192 A. The
capacitance of the sorbent / sorbate system decreases with increasing
frequency of the field – so-called normal dispersion [6.6]. However, the
capacitance increases with increasing gas pressure, i. e. amount of gas
adsorbed. This is due to the increase of the number of admolecules within the
system each of which having an electric dipole moment
Also it should be noted that the changes in the capacitance with changing gas
pressure or amount of adsorbed are much larger for low frequencies
than for higher ones This gives an indication of
which frequency should be used to observe breakthrough curves or to check
the state of a porous sorbent in industrial adsorption processes [6.14, 6.15].