Page 312 - Gas Adsorption Equilibria
P. 312
298 Chapter 6
Calculations of the dielectric polarizability of the adsorbed nitrogen
from the data presented in Fig. 6.6 indicate that admolecules are in an
“intermediate state” between gas and liquid, cp. Sects. 2.2, 3.2.
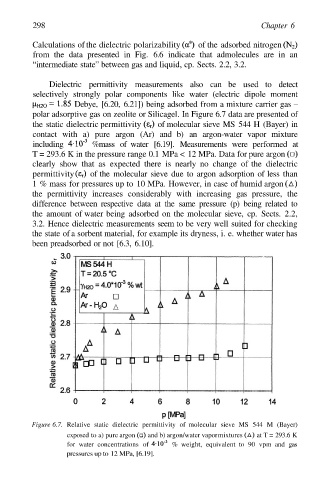
Dielectric permittivity measurements also can be used to detect
selectively strongly polar components like water (electric dipole moment
Debye, [6.20, 6.21]) being adsorbed from a mixture carrier gas –
polar adsorptive gas on zeolite or Silicagel. In Figure 6.7 data are presented of
the static dielectric permittivity of molecular sieve MS 544 H (Bayer) in
contact with a) pure argon (Ar) and b) an argon-water vapor mixture
including %mass of water [6.19]. Measurements were performed at
T = 293.6 K in the pressure range 0.1 MPa < 12 MPa. Data for pure argon
clearly show that as expected there is nearly no change of the dielectric
permittivity of the molecular sieve due to argon adsorption of less than
1 % mass for pressures up to 10 MPa. However, in case of humid argon
the permittivity increases considerably with increasing gas pressure, the
difference between respective data at the same pressure (p) being related to
the amount of water being adsorbed on the molecular sieve, cp. Sects. 2.2,
3.2. Hence dielectric measurements seem to be very well suited for checking
the state of a sorbent material, for example its dryness, i. e. whether water has
been preadsorbed or not [6.3, 6.10].
Figure 6.7. Relative static dielectric permittivity of molecular sieve MS 544 M (Bayer)
exposed to a) pure argon and b) argon/water vapormixtures at T = 293.6 K
for water concentrations of % weight, equivalent to 90 vpm and gas
pressures up to 12 MPa, [6.19].