Page 33 - Gas Adsorption Equilibria
P. 33
1. Basic Concepts 19
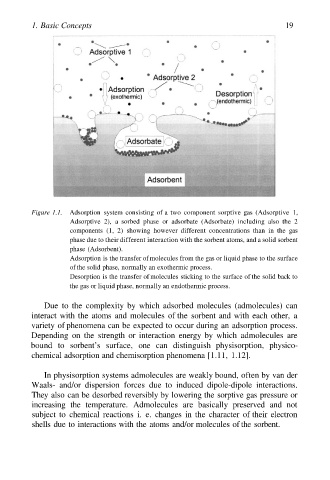
Figure 1.1. Adsorption system consisting of a two component sorptive gas (Adsorptive 1,
Adsorptive 2), a sorbed phase or adsorbate (Adsorbate) including also the 2
components (1, 2) showing however different concentrations than in the gas
phase due to their different interaction with the sorbent atoms, and a solid sorbent
phase (Adsorbent).
Adsorption is the transfer of molecules from the gas or liquid phase to the surface
of the solid phase, normally an exothermic process.
Desorption is the transfer of molecules sticking to the surface of the solid back to
the gas or liquid phase, normally an endothermic process.
Due to the complexity by which adsorbed molecules (admolecules) can
interact with the atoms and molecules of the sorbent and with each other, a
variety of phenomena can be expected to occur during an adsorption process.
Depending on the strength or interaction energy by which admolecules are
bound to sorbent’s surface, one can distinguish physisorption, physico-
chemical adsorption and chemisorption phenomena [1.11, 1.12].
In physisorption systems admolecules are weakly bound, often by van der
Waals- and/or dispersion forces due to induced dipole-dipole interactions.
They also can be desorbed reversibly by lowering the sorptive gas pressure or
increasing the temperature. Admolecules are basically preserved and not
subject to chemical reactions i. e. changes in the character of their electron
shells due to interactions with the atoms and/or molecules of the sorbent.