Page 37 - Gas Adsorption Equilibria
P. 37
1. Basic Concepts 23
Adsorbates, i. e. sets of adsorbed molecules, can assume, from a
macroscopic point of view, many different types of thermodynamic phases.
The structure of these phases mainly depends on the number of molecules
adsorbed, on the strength of interactions between admolecules and sorbent
atoms and on the geometry of the surface of the sorbent material or,
equivalently, on its pore size distribution.
At low adsorption loads admolecules often form type of lattice gas on the
surface of the sorbent, i. e. admolecules mainly are isolated from each other
but jump around from one adsorption site to another. An example for such a
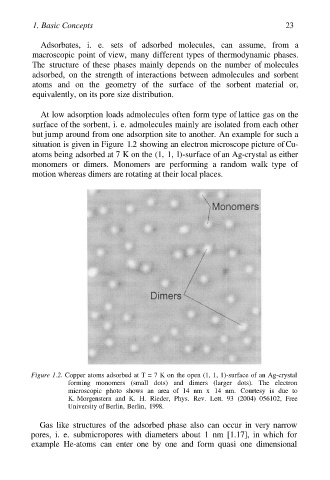
situation is given in Figure 1.2 showing an electron microscope picture of Cu-
atoms being adsorbed at 7 K on the (1, 1, l)-surface of an Ag-crystal as either
monomers or dimers. Monomers are performing a random walk type of
motion whereas dimers are rotating at their local places.
Figure 1.2. Copper atoms adsorbed at T = 7 K on the open (1, 1, 1)-surface of an Ag-crystal
forming monomers (small dots) and dimers (larger dots). The electron
microscopic photo shows an area of 14 nm x 14 nm. Courtesy is due to
K. Morgenstern and K. H. Rieder, Phys. Rev. Lett. 93 (2004) 056102, Free
University of Berlin, Berlin, 1998.
Gas like structures of the adsorbed phase also can occur in very narrow
pores, i. e. submicropores with diameters about 1 nm [1.17], in which for
example He-atoms can enter one by one and form quasi one dimensional