Page 36 - Gas Adsorption Equilibria
P. 36
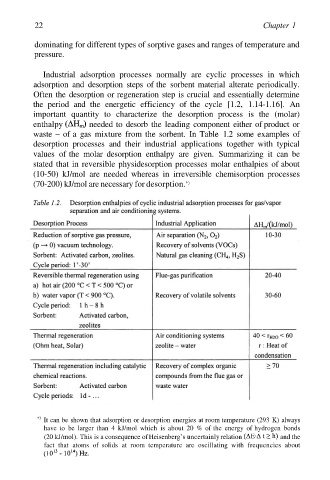
22 Chapter 1
dominating for different types of sorptive gases and ranges of temperature and
pressure.
Industrial adsorption processes normally are cyclic processes in which
adsorption and desorption steps of the sorbent material alterate periodically.
Often the desorption or regeneration step is crucial and essentially determine
the period and the energetic efficiency of the cycle [1.2, 1.14-1.16]. An
important quantity to characterize the desorption process is the (molar)
enthalpy needed to desorb the leading component either of product or
waste – of a gas mixture from the sorbent. In Table 1.2 some examples of
desorption processes and their industrial applications together with typical
values of the molar desorption enthalpy are given. Summarizing it can be
stated that in reversible physidesorption processes molar enthalpies of about
(10-50) kJ/mol are needed whereas in irreversible chemisorption processes
(70-200) kJ/mol are necessary for desorption. *)
* ) It can be shown that adsorption or desorption energies at room temperature (293 K) always
have to be larger than 4 kJ/mol which is about 20 % of the energy of hydrogen bonds
(20 kJ/mol). This is a consequence of Heisenberg’s uncertainly relation and the
fact that atoms of solids at room temperature are oscillating with frequencies about