Page 47 - Gas Adsorption Equilibria
P. 47
1. Basic Concepts 33
Here is the surface tension of mercury which on principle is a
pressure and temperature dependent quantity , is the contact angle
* )
between the mercury meniscus and the pore wall. Though the exact value of
this parameter normally is unknown, a practical value of 140° turned out to
lead to physically reasonable results in many cases and hence is recommended
for practical use [l.44].
The total volume of mercury penetrating the pores of the material
at pressure p leads via equation (1.2) to the integral volume of all pores
with radii larger than i. e. By differentiation to
the pore radius r this yields the differential pore size distribution of the
material. This method is valuable to investigate macro- and mesopores
(IUPAC, cp. Sect. 3), but not for micropores, i. e. it is limited to pore
radii r > 1 nm.
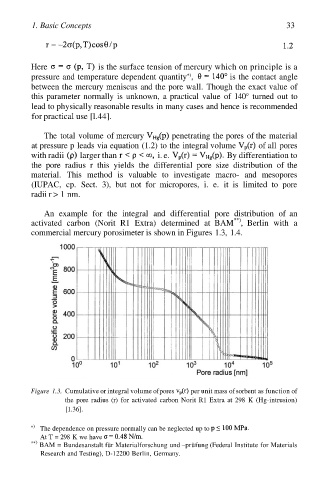
An example for the integral and differential pore distribution of an
**)
activated carbon (Norit R1 Extra) determined at BAM , Berlin with a
commercial mercury porosimeter is shown in Figures 1.3, 1.4.
Figure 1.3. Cumulative or integral volume of pores per unit mass of sorbent as function of
the pore radius (r) for activated carbon Norit R1 Extra at 298 K (Hg-intrusion)
[1.36].
* ) The dependence on pressure normally can be neglected up to
At T = 298 K we have
** ) BAM = Bundesanstalt für Materialforschung und –prüfung (Federal Institute for Materials
Research and Testing), D-12200 Berlin, Germany.