Page 51 - Gas Adsorption Equilibria
P. 51
1. Basic Concepts 37
Figure 1.6. As can be seen, resulting values of the specific volume of the
AC as “seen” by the He-molecules depend on the gas pressure. Differences
make up to 2 % in the pressure range considered. Several measurement
procedures have been used. Data presented in the figure are statistical
averages of 10, 10, 25, 99 separate measurements [1.48].
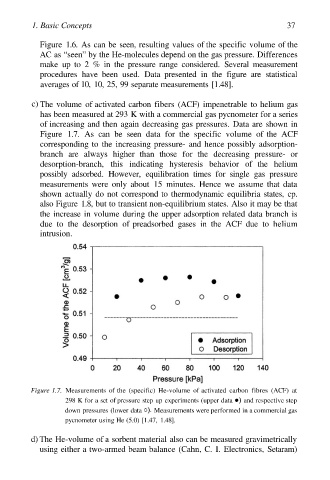
c) The volume of activated carbon fibers (ACF) impenetrable to helium gas
has been measured at 293 K with a commercial gas pycnometer for a series
of increasing and then again decreasing gas pressures. Data are shown in
Figure 1.7. As can be seen data for the specific volume of the ACF
corresponding to the increasing pressure- and hence possibly adsorption-
branch are always higher than those for the decreasing pressure- or
desorption-branch, this indicating hysteresis behavior of the helium
possibly adsorbed. However, equilibration times for single gas pressure
measurements were only about 15 minutes. Hence we assume that data
shown actually do not correspond to thermodynamic equilibria states, cp.
also Figure 1.8, but to transient non-equilibrium states. Also it may be that
the increase in volume during the upper adsorption related data branch is
due to the desorption of preadsorbed gases in the ACF due to helium
intrusion.
Figure 1.7. Measurements of the (specific) He-volume of activated carbon fibres (ACF) at
298 K for a set of pressure step up experiments (upper data and respective step
down pressures (lower data Measurements were performed in a commercial gas
pycnometer using He (5.0) [1.47, 1.48].
d) The He-volume of a sorbent material also can be measured gravimetrically
using either a two-armed beam balance (Cahn, C. I. Electronics, Setaram)