Page 55 - Gas Adsorption Equilibria
P. 55
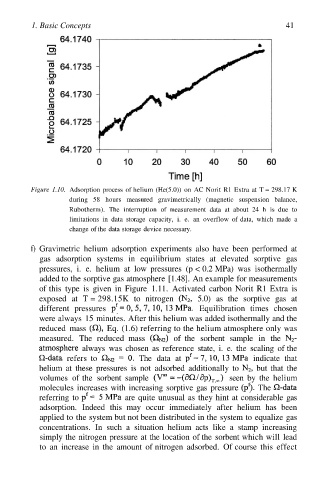
1. Basic Concepts 41
Figure 1.10. Adsorption process of helium (He(5.0)) on AC Norit R1 Extra at T = 298.17 K
during 58 hours measured gravimetrically (magnetic suspension balance,
Rubotherm). The interruption of measurement data at about 24 h is due to
limitations in data storage capacity, i. e. an overflow of data, which made a
change of the data storage device necessary.
f) Gravimetric helium adsorption experiments also have been performed at
gas adsorption systems in equilibrium states at elevated sorptive gas
pressures, i. e. helium at low pressures (p < 0.2 MPa) was isothermally
added to the sorptive gas atmosphere [1.48]. An example for measurements
of this type is given in Figure 1.11. Activated carbon Norit R1 Extra is
exposed at T = 298.15K to nitrogen 5.0) as the sorptive gas at
different pressures Equilibration times chosen
were always 15 minutes. After this helium was added isothermally and the
reduced mass Eq. (1.6) referring to the helium atmosphere only was
measured. The reduced mass of the sorbent sample in the
always was chosen as reference state, i. e. the scaling of the
refers to The data at indicate that
helium at these pressures is not adsorbed additionally to but that the
volumes of the sorbent sample seen by the helium
molecules increases with increasing sorptive gas pressure The
referring to are quite unusual as they hint at considerable gas
adsorption. Indeed this may occur immediately after helium has been
applied to the system but not been distributed in the system to equalize gas
concentrations. In such a situation helium acts like a stamp increasing
simply the nitrogen pressure at the location of the sorbent which will lead
to an increase in the amount of nitrogen adsorbed. Of course this effect