Page 54 - Gas Adsorption Equilibria
P. 54
40 Chapter 1
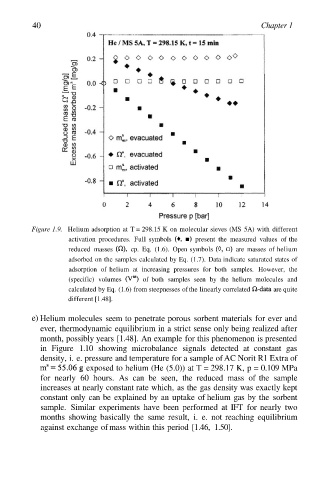
Figure 1.9. Helium adsorption at T = 298.15 K on molecular sieves (MS 5A) with different
activation procedures. Full symbols present the measured values of the
reduced masses cp. Eq. (1.6). Open symbols are masses of helium
adsorbed on the samples calculated by Eq. (1.7). Data indicate saturated states of
adsorption of helium at increasing pressures for both samples. However, the
(specific) volumes of both samples seen by the helium molecules and
calculated by Eq. (1.6) from steepnesses of the linearly correlated are quite
different [1.48].
e) Helium molecules seem to penetrate porous sorbent materials for ever and
ever, thermodynamic equilibrium in a strict sense only being realized after
month, possibly years [1.48]. An example for this phenomenon is presented
in Figure 1.10 showing microbalance signals detected at constant gas
density, i. e. pressure and temperature for a sample of AC Norit R1 Extra of
exposed to helium (He (5.0)) at T = 298.17 K, p = 0.109 MPa
for nearly 60 hours. As can be seen, the reduced mass of the sample
increases at nearly constant rate which, as the gas density was exactly kept
constant only can be explained by an uptake of helium gas by the sorbent
sample. Similar experiments have been performed at IFT for nearly two
months showing basically the same result, i. e. not reaching equilibrium
against exchange of mass within this period [1.46, 1.50].