Page 53 - Gas Adsorption Equilibria
P. 53
1. Basic Concepts 39
has a density which is about twice its value in the
corresponding bulk liquid state at the same temperature [1.17, 1.20].
Gravimetric measurements of helium adsorption are very sensitive to the
actual state of the sorbent material, i. e. to whether it has been activated or
not. Also presorbed gases can change the helium adsorption isotherm
considerably. An example for this is given in Figure 1.9 showing data of two
samples of molecular sieves (MS5A) one of which having been prepared by
evacuation of the adsorption chamber at ambient temperature (293 K), the
other having been activated in vacuum at elevated temperature (573 K) for
several hours [1.48]. In both cases the experimental data of the reduced
masses cp. Eq. (1.6), for higher pressures can be linearly
correlated as functions of either the gas pressure (p) or the density
and hence indicate constant mass of helium adsorbed in the
initial period of the experiment, i. e. at very low gas pressures, cp. Eq. (1.7).
The respective data are shown as symbols indicating for a
saturated state of adsorption
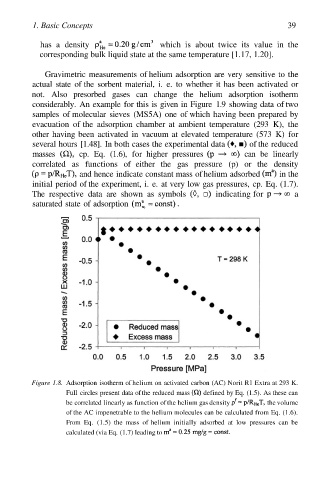
Figure 1.8. Adsorption isotherm of helium on activated carbon (AC) Norit R1 Extra at 293 K.
Full circles present data of the reduced mass defined by Eq. (1.5). As these can
be correlated linearly as function of the helium gas density the volume
of the AC impenetrable to the helium molecules can be calculated from Eq. (1.6).
From Eq. (1.5) the mass of helium initially adsorbed at low pressures can be
calculated (via Eq. (1.7) leading to