Page 60 - Gas Adsorption Equilibria
P. 60
46 Chapter 1
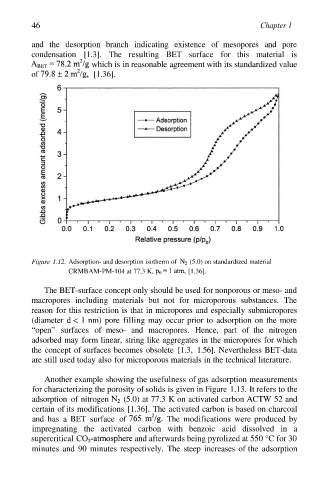
and the desorption branch indicating existence of mesopores and pore
condensation [1.3]. The resulting BET surface for this material is
which is in reasonable agreement with its standardized value
of [1.36].
Figure 1.12. Adsorption- and desorption isotherm of (5.0) on standardized material
CRMBAM-PM-104 at 77.3 K, [1.36].
The BET-surface concept only should be used for nonporous or meso- and
macropores including materials but not for microporous substances. The
reason for this restriction is that in micropores and especially submicropores
(diameter d < 1 nm) pore filling may occur prior to adsorption on the more
“open” surfaces of meso- and macropores. Hence, part of the nitrogen
adsorbed may form linear, string like aggregates in the micropores for which
the concept of surfaces becomes obsolete [1.3, 1.56]. Nevertheless BET-data
are still used today also for microporous materials in the technical literature.
Another example showing the usefulness of gas adsorption measurements
for characterizing the porosity of solids is given in Figure 1.13. It refers to the
adsorption of nitrogen (5.0) at 77.3 K on activated carbon ACTW 52 and
certain of its modifications [1.36]. The activated carbon is based on charcoal
and has a BET surface of The modifications were produced by
impregnating the activated carbon with benzoic acid dissolved in a
supercritical and afterwards being pyrolized at 550 °C for 30
minutes and 90 minutes respectively. The steep increases of the adsorption