Page 64 - Gas Adsorption Equilibria
P. 64
50 Chapter 1
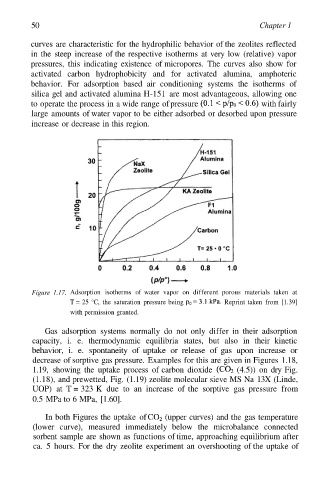
curves are characteristic for the hydrophilic behavior of the zeolites reflected
in the steep increase of the respective isotherms at very low (relative) vapor
pressures, this indicating existence of micropores. The curves also show for
activated carbon hydrophobicity and for activated alumina, amphoteric
behavior. For adsorption based air conditioning systems the isotherms of
silica gel and activated alumina H-151 are most advantageous, allowing one
to operate the process in a wide range of pressure with fairly
large amounts of water vapor to be either adsorbed or desorbed upon pressure
increase or decrease in this region.
Figure 1.17. Adsorption isotherms of water vapor on different porous materials taken at
T = 25 °C, the saturation pressure being Reprint taken from [1.39]
with permission granted.
Gas adsorption systems normally do not only differ in their adsorption
capacity, i. e. thermodynamic equilibria states, but also in their kinetic
behavior, i. e. spontaneity of uptake or release of gas upon increase or
decrease of sorptive gas pressure. Examples for this are given in Figures 1.18,
1.19, showing the uptake process of carbon dioxide (4.5)) on dry Fig.
(1.18), and prewetted, Fig. (1.19) zeolite molecular sieve MS Na 13X (Linde,
UOP) at T = 323 K due to an increase of the sorptive gas pressure from
0.5 MPa to 6 MPa, [1.60].
In both Figures the uptake of (upper curves) and the gas temperature
(lower curve), measured immediately below the microbalance connected
sorbent sample are shown as functions of time, approaching equilibrium after
ca. 5 hours. For the dry zeolite experiment an overshooting of the uptake of