Page 61 - Gas Adsorption Equilibria
P. 61
1. Basic Concepts 47
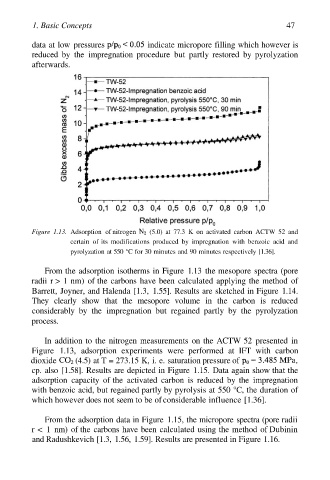
data at low pressures indicate micropore filling which however is
reduced by the impregnation procedure but partly restored by pyrolyzation
afterwards.
Figure 1.13. Adsorption of nitrogen (5.0) at 77.3 K on activated carbon ACTW 52 and
certain of its modifications produced by impregnation with benzoic acid and
pyrolyzation at 550 °C for 30 minutes and 90 minutes respectively [1.36].
From the adsorption isotherms in Figure 1.13 the mesopore spectra (pore
radii r > 1 nm) of the carbons have been calculated applying the method of
Barrett, Joyner, and Halenda [1.3, 1.55]. Results are sketched in Figure 1.14.
They clearly show that the mesopore volume in the carbon is reduced
considerably by the impregnation but regained partly by the pyrolyzation
process.
In addition to the nitrogen measurements on the ACTW 52 presented in
Figure 1.13, adsorption experiments were performed at IFT with carbon
dioxide (4.5) at T = 273.15 K, i. e. saturation pressure of
cp. also [1.58]. Results are depicted in Figure 1.15. Data again show that the
adsorption capacity of the activated carbon is reduced by the impregnation
with benzoic acid, but regained partly by pyrolysis at 550 °C, the duration of
which however does not seem to be of considerable influence [1.36].
From the adsorption data in Figure 1.15, the micropore spectra (pore radii
r < 1 nm) of the carbons have been calculated using the method of Dubinin
and Radushkevich [1.3, 1.56, 1.59]. Results are presented in Figure 1.16.