Page 65 - Gas Adsorption Equilibria
P. 65
1. Basic Concepts 51
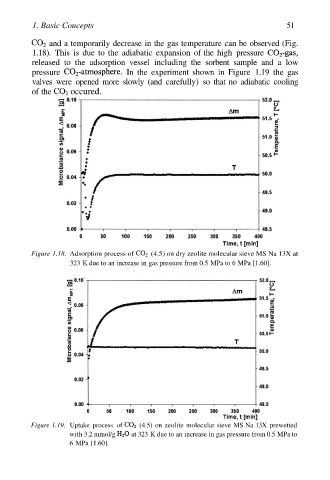
and a temporarily decrease in the gas temperature can be observed (Fig.
1.18). This is due to the adiabatic expansion of the high pressure
released to the adsorption vessel including the sorbent sample and a low
pressure In the experiment shown in Figure 1.19 the gas
valves were opened more slowly (and carefully) so that no adiabatic cooling
of the occured.
Figure 1.18. Adsorption process of (4.5) on dry zeolite molecular sieve MS Na 13X at
323 K due to an increase in gas pressure from 0.5 MPa to 6 MPa [1.60].
Figure 1.19. Uptake process of (4.5) on zeolite molecular sieve MS Na 13X prewetted
with 3.2 mmol/g at 323 K due to an increase in gas pressure from 0.5 MPa to
6 MPa [1.60].