Page 68 - Gas Adsorption Equilibria
P. 68
54 Chapter 1
This is the difference between and the mass of the sorptive fluid that
would be included in the volume if there were no (attractive) surface forces
of the sorbent material, i. e. we have in view of Eq. (1.14), [1.1-1.3]
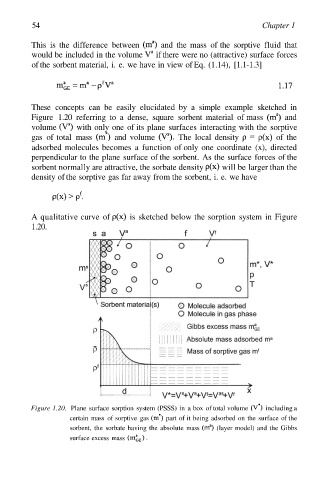
These concepts can be easily elucidated by a simple example sketched in
Figure 1.20 referring to a dense, square sorbent material of mass and
volume with only one of its plane surfaces interacting with the sorptive
gas of total mass and volume The local density of the
adsorbed molecules becomes a function of only one coordinate (x), directed
perpendicular to the plane surface of the sorbent. As the surface forces of the
sorbent normally are attractive, the sorbate density will be larger than the
density of the sorptive gas far away from the sorbent, i. e. we have
A qualitative curve of is sketched below the sorption system in Figure
1.20.
Figure 1.20. Plane surface sorption system (PSSS) in a box of total volume including a
certain mass of sorptive gas part of it being adsorbed on the surface of the
sorbent, the sorbate having the absolute mass (layer model) and the Gibbs
surface excess mass