Page 36 - gas transport in porous media
P. 36
Chapter 3: Vapor Transport Processes
120
100 29
80
Pressure (kPa) 60 TCE
40
Water
20
0
–20 0 20 40 60 80 100 120
Temperature (°C)
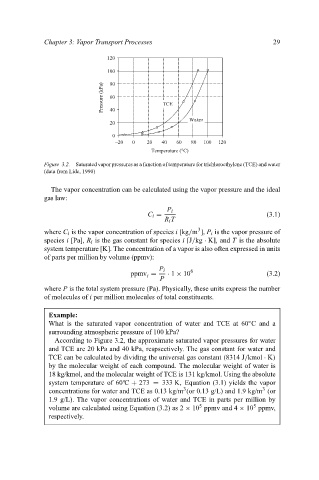
Figure 3.2. Saturated vapor pressures as a function of temperature for trichloroethylene (TCE) and water
(data from Lide, 1990)
The vapor concentration can be calculated using the vapor pressure and the ideal
gas law:
P i
C i = (3.1)
R i T
3
where C i is the vapor concentration of species i [kg/m ], P i is the vapor pressure of
species i [Pa], R i is the gas constant for species i [J/kg · K], and T is the absolute
system temperature [K]. The concentration of a vapor is also often expressed in units
of parts per million by volume (ppmv):
P i 6
ppmv = · 1 × 10 (3.2)
i
P
where P is the total system pressure (Pa). Physically, these units express the number
of molecules of i per million molecules of total constituents.
Example:
◦
What is the saturated vapor concentration of water and TCE at 60 C and a
surrounding atmospheric pressure of 100 kPa?
According to Figure 3.2, the approximate saturated vapor pressures for water
and TCE are 20 kPa and 40 kPa, respsectively. The gas constant for water and
TCE can be calculated by dividing the universal gas constant (8314 J/kmol · K)
by the molecular weight of each compound. The molecular weight of water is
18 kg/kmol, and the molecular weight of TCE is 131 kg/kmol. Using the absolute
◦
system temperature of 60 C + 273 = 333 K, Equation (3.1) yields the vapor
3
3
concentrations for water and TCE as 0.13 kg/m (or 0.13 g/L) and 1.9 kg/m (or
1.9 g/L). The vapor concentrations of water and TCE in parts per million by
5
5
volume are calculated using Equation (3.2) as 2 × 10 ppmv and 4 × 10 ppmv,
respectively.