Page 40 - gas transport in porous media
P. 40
Chapter 3: Vapor Transport Processes
10.0
9.0 33
8.0
7.0
P v / P sat 6.0
5.0
4.0
TCE
3.0
2.0
1.0 Water
0.0
1.0E–09 1.0E–08 1.0E–07 1.0E–06
Convex radius of curvature (m)
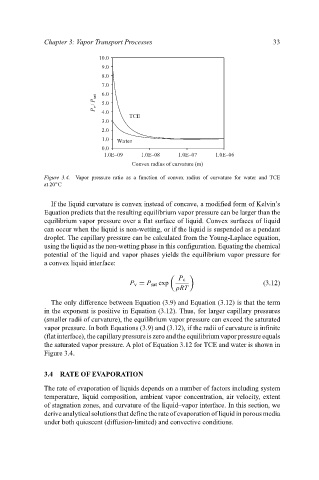
Figure 3.4. Vapor pressure ratio as a function of convex radius of curvature for water and TCE
◦
at 20 C
If the liquid curvature is convex instead of concave, a modified form of Kelvin’s
Equation predicts that the resulting equilibrium vapor pressure can be larger than the
equilibrium vapor pressure over a flat surface of liquid. Convex surfaces of liquid
can occur when the liquid is non-wetting, or if the liquid is suspended as a pendant
droplet. The capillary pressure can be calculated from the Young-Laplace equation,
using the liquid as the non-wetting phase in this configuration. Equating the chemical
potential of the liquid and vapor phases yields the equilibrium vapor pressure for
a convex liquid interface:
P c
P v = P sat exp (3.12)
ρRT
The only difference between Equation (3.9) and Equation (3.12) is that the term
in the exponent is positive in Equation (3.12). Thus, for larger capillary pressures
(smaller radii of curvature), the equilibrium vapor pressure can exceed the saturated
vapor pressure. In both Equations (3.9) and (3.12), if the radii of curvature is infinite
(flat interface), the capillary pressure is zero and the equilibrium vapor pressure equals
the saturated vapor pressure. A plot of Equation 3.12 for TCE and water is shown in
Figure 3.4.
3.4 RATE OF EVAPORATION
The rate of evaporation of liquids depends on a number of factors including system
temperature, liquid composition, ambient vapor concentration, air velocity, extent
of stagnation zones, and curvature of the liquid–vapor interface. In this section, we
derive analytical solutions that define the rate of evaporation of liquid in porous media
under both quiescent (diffusion-limited) and convective conditions.