Page 58 - gas transport in porous media
P. 58
Chapter 4: Solid/Gas Partitioning
12
Monolayer of Alumina 51
10 water molecules Alumina with humic acid
Iron oxide
Montmorillonite
8 Kaolinite
ln ( K ' d - K d /K H ) 6 4 Limit of application
Dissolution of TCE
for Henry's law
2
0
–2
0 2 4 6 8 10 12 14 16 18 20
Number of layers of water molecules
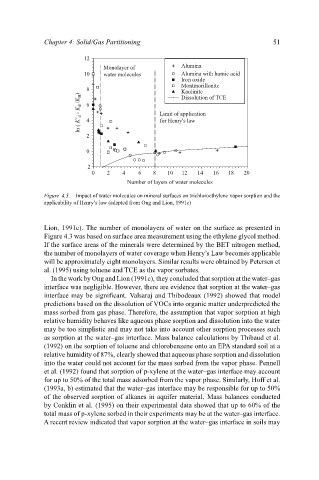
Figure 4.3. Impact of water molecules on mineral surfaces on trichloroethylene vapor sorption and the
applicability of Henry’s law (adapted from Ong and Lion, 1991c)
Lion, 1991c). The number of monolayers of water on the surface as presented in
Figure 4.3 was based on surface area measurement using the ethylene glycol method.
If the surface areas of the minerals were determined by the BET nitrogen method,
the number of monolayers of water coverage when Henry’s Law becomes applicable
will be approximately eight monolayers. Similar results were obtained by Petersen et
al. (1995) using toluene and TCE as the vapor sorbates.
In the work by Ong and Lion (1991c), they concluded that sorption at the water–gas
interface was negligible. However, there are evidence that sorption at the water–gas
interface may be significant. Valsaraj and Thibodeaux (1992) showed that model
predictions based on the dissolution of VOCs into organic matter underpredicted the
mass sorbed from gas phase. Therefore, the assumption that vapor sorption at high
relative humidity behaves like aqueous phase sorption and dissolution into the water
may be too simplistic and may not take into account other sorption processes such
as sorption at the water–gas interface. Mass balance calculations by Thibaud et al.
(1992) on the sorption of toluene and chlorobenzene onto an EPA standard soil at a
relative humidity of 87%, clearly showed that aqueous phase sorption and dissolution
into the water could not account for the mass sorbed from the vapor phase. Pennell
et al. (1992) found that sorption of p-xylene at the water–gas interface may account
for up to 50% of the total mass adsorbed from the vapor phase. Similarly, Hoff et al.
(1993a, b) estimated that the water–gas interface may be responsible for up to 50%
of the observed sorption of alkanes in aquifer material. Mass balances conducted
by Conklin et al. (1995) on their experimental data showed that up to 60% of the
total mass of p-xylene sorbed in their experiments may be at the water–gas interface.
A recent review indicated that vapor sorption at the water–gas interface in soils may