Page 55 - gas transport in porous media
P. 55
48
(a) Water molecule (b) Ong
Vapor phase Organic molecule
Vapor phase
4
5
2
5
Water
1 6
3
Solid
Solid
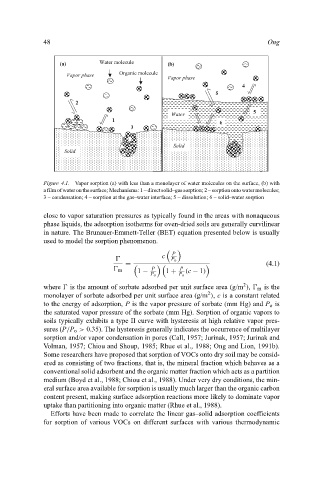
Figure 4.1. Vapor sorption (a) with less than a monolayer of water molecules on the surface, (b) with
a film of water on the surface; Mechanisms: 1 – direct solid–gas sorption; 2 – sorption onto water molecules;
3 – condensation; 4 – sorption at the gas–water interface; 5 – dissolution; 6 – solid–water sorption
close to vapor saturation pressures as typically found in the areas with nonaqueous
phase liquids, the adsorption isotherms for oven-dried soils are generally curvilinear
in nature. The Brunauer-Emmett-Teller (BET) equation presented below is usually
used to model the sorption phenomenon.
P
c P o
(4.1)
=
m P P
1 − 1 + (c − 1)
P o P o
2
where is the amount of sorbate adsorbed per unit surface area (g/m ), m is the
2
monolayer of sorbate adsorbed per unit surface area (g/m ), c is a constant related
to the energy of adsorption, P is the vapor pressure of sorbate (mm Hg) and P o is
the saturated vapor pressure of the sorbate (mm Hg). Sorption of organic vapors to
soils typically exhibits a type II curve with hysteresis at high relative vapor pres-
sures (P/P o > 0.35). The hysteresis generally indicates the occurrence of multilayer
sorption and/or vapor condensation in pores (Call, 1957; Jurinak, 1957; Jurinak and
Volman, 1957; Chiou and Shoup, 1985; Rhue et al., 1988; Ong and Lion, 1991b).
Some researchers have proposed that sorption of VOCs onto dry soil may be consid-
ered as consisting of two fractions, that is, the mineral fraction which behaves as a
conventional solid adsorbent and the organic matter fraction which acts as a partition
medium (Boyd et al., 1988; Chiou et al., 1988). Under very dry conditions, the min-
eral surface area available for sorption is usually much larger than the organic carbon
content present, making surface adsorption reactions more likely to dominate vapor
uptake than partitioning into organic matter (Rhue et al., 1988).
Efforts have been made to correlate the linear gas–solid adsorption coefficients
for sorption of various VOCs on different surfaces with various thermodynamic