Page 51 - gas transport in porous media
P. 51
44
30 + All Gas Ho
All Gas
Cumulative Drawdown (mm 3 ) 20 + + + + 6.0 mm LI
25
5.6 mm LI
7.5 mm LI
15
7.5 mm LI
10
8.0 mm LI
0 5 + + + + + + + 7.8 mm LI
0 10 20 30 40 50 8.9 mm LI
Time (hr)
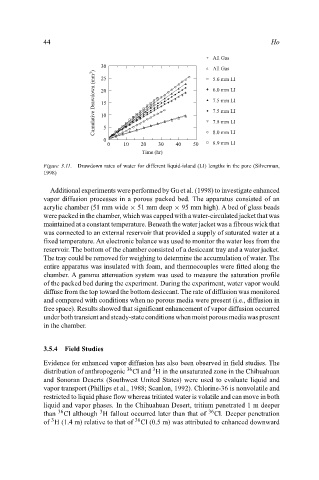
Figure 3.11. Drawdown rates of water for different liquid-island (LI) lengths in the pore (Silverman,
1998)
Additional experiments were performed by Gu et al. (1998) to investigate enhanced
vapor diffusion processes in a porous packed bed. The apparatus consisted of an
acrylic chamber (51 mm wide × 51 mm deep × 95 mm high). A bed of glass beads
were packed in the chamber, which was capped with a water-circulated jacket that was
maintained at a constant temperature. Beneath the water jacket was a fibrous wick that
was connected to an external reservoir that provided a supply of saturated water at a
fixed temperature. An electronic balance was used to monitor the water loss from the
reservoir. The bottom of the chamber consisted of a desiccant tray and a water jacket.
The tray could be removed for weighing to determine the accumulation of water. The
entire apparatus was insulated with foam, and thermocouples were fitted along the
chamber. A gamma attenuation system was used to measure the saturation profile
of the packed bed during the experiment. During the experiment, water vapor would
diffuse from the top toward the bottom desiccant. The rate of diffusion was monitored
and compared with conditions when no porous media were present (i.e., diffusion in
free space). Results showed that significant enhancement of vapor diffusion occurred
under both transient and steady-state conditions when moist porous media was present
in the chamber.
3.5.4 Field Studies
Evidence for enhanced vapor diffusion has also been observed in field studies. The
3
distribution of anthropogenic 36 Cl and H in the unsaturated zone in the Chihuahuan
and Sonoran Deserts (Southwest United States) were used to evaluate liquid and
vapor transport (Phillips et al., 1988; Scanlon, 1992). Chlorine-36 is nonvolatile and
restricted to liquid phase flow whereas tritiated water is volatile and can move in both
liquid and vapor phases. In the Chihuahuan Desert, tritium penetrated 1 m deeper
3
than 36 Cl although H fallout occurred later than that of 36 Cl. Deeper penetration
3
of H (1.4 m) relative to that of 36 Cl (0.5 m) was attributed to enhanced downward