Page 48 - gas transport in porous media
P. 48
Chapter 3: Vapor Transport Processes
difference across the liquid island and the tortuosity, τ (actual path length divided by
linear path length, L): 41
M v ∂P v P v T
m v,Fick = DA p − (3.36)
RT ∂T T τL
where M v is the molecular weight of water [18 kg/kmol], R is the ideal gas constant
[8300 J/kmol-K], and P v is the saturated vapor pressure of water [Pa].
3.5.1.3 Ratio
Equation (3.34) is divided by equation (3.36) to obtain a ratio of the mass flow rates
due to enhanced vapor diffusion mechanisms and Fickian diffusion:
m v,evd A t λ l RT τ
=
(3.37)
m v,Fick A p h fg D M v ∂P v − P v
∂T T
A value of this ratio on the order of one or more indicates that enhanced vapor
phase diffusion can be significant relative to Fickian diffusion in transporting water
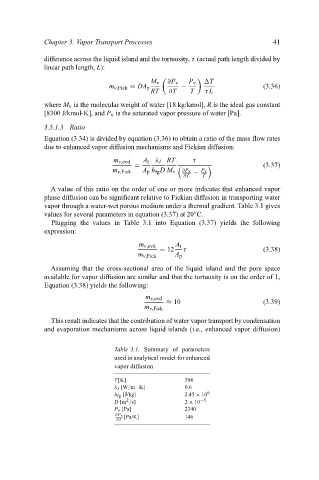
vapor through a water-wet porous medium under a thermal gradient. Table 3.1 gives
◦
values for several parameters in equation (3.37) at 20 C.
Plugging the values in Table 3.1 into Equation (3.37) yields the following
expression:
m v,evd A t
= 12 τ (3.38)
m v,Fick A p
Assuming that the cross-sectional area of the liquid island and the pore space
available for vapor diffusion are similar and that the tortuosity is on the order of 1,
Equation (3.38) yields the following:
m v,evd
≈ 10 (3.39)
m v,Fick
This result indicates that the contribution of water vapor transport by condensation
and evaporation mechanisms across liquid islands (i.e., enhanced vapor diffusion)
Table 3.1. Summary of parameters
used in analytical model for enhanced
vapor diffusion
T[K] 298
λ l [W/m · K] 0.6
h fg [J/kg] 2.45 × 10 6
2 −5
D [m /s] 2 × 10
P v [Pa] 2340
∂P v [Pa/K] 146
∂T