Page 217 - Geochemistry of Oil Field Waters
P. 217
204 ORIGIN OF OILFIELD WATERS
Deffeyes et al. (1964) proposed that dolomitization of limestone results
from the evaporation of sea water and precipitation of gypsum causing the
ratio of magnesium to calcium in the water to increase. The concentrated
water flows downward, because it is more dense, into the underlying sedi-
ments where it reacts with limestone to form dolomite.
Modern evaporite deposits are thin and cover relatively small areas of the
earth; however, the ancient environments indicate that these depositions
were widespread in the United States (Krumbein, 1951) and in the world
(Lotze, 1938). The majority of the major evaporite bodies are of marine
origin, and range in age from Cambrian to Tertiary. They form in arid marine
climates where water lost by evaporation equals or exceeds that supplied by
rainfall, rivers, or the open sea. They also form in deep-water environment
(Brongersma-Sanders, 1971 ).
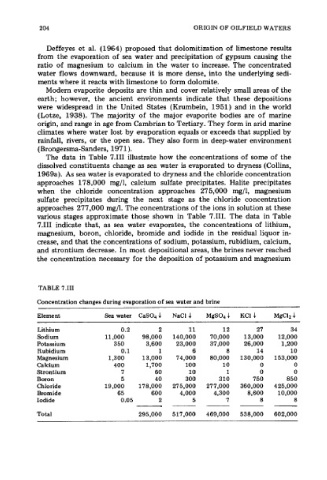
The data in Table 7.111 illustrate how the concentrations of some of the
dissolved constituents change as sea water is evaporated to dryness (Collins,
1969a). As sea water is evaporated to dryness and the chloride concentration
approaches 178,000 mg/l, calcium sulfate precipitates. Halite precipitates
when the chloride concentration approaches 27 5,000 mg/l, magnesium
sulfate precipitates during the next stage as the chloride concentration
approaches 277,000 mg/l. The concentrations of the ions in solution at these
various stages approximate those shown in Table 7.111. The data in Table
7.111 indicate that, as sea water evaporates, the concentrations of lithium,
magnesium, boron, chloride, bromide and iodide in the residual liquor in-
crease, and that the concentrations of sodium, potassium, rubidium, calcium,
and strontium decrease. In most depositional areas, the brines never reached
the concentration necessary for the deposition of potassium and magnesium
TABLE 7.111
Concentration changes during evaporation of sea water and brine
Element Sea water CaS04.l NaCl 4 MgS04.l KCI 4 MgClz.1
Lithium 0.2 2 11 12 27 34
Sodium 11,000 98,000 140,000 70,000 13,000 12,000
Potassium 350 3,600 23,000 37,000 26,000 1,200
Rubidium 0.1 1 6 8 14 10
Magnesium 1,300 13,000 74,000 80,000 130,000 153,000
Calcium 400 1,700 100 10 0 0
Strontium 7 60 10 1 0 0
Boron 5 40 300 310 750 850
Chloride 19,000 178,000 275,000 27 7,000 360,000 425,000
Bromide 65 600 4,000 4,300 8,600 10,000
Iodide 0.05 2 5 7 8 8
Total 295,000 517,000 469,000 538,000 602,000