Page 57 - Geochemistry of Oil Field Waters
P. 57
TITRIMETRIC METHODS 45
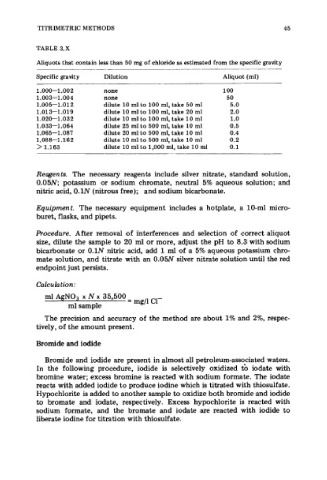
TABLE 3.X
Aliquots that contain less than 50 mg of chloride as estimated from the specific gravity
Specific gravity Dilution Aliquot (ml)
1.000-1.002 none 100
1.003-1.004 none 50
1.005-1.012 dilute 10 ml to 100 ml, take 50 ml 5.0
1.01 3-1.019 dilute 10 ml to 100 ml, take 20 ml 2.0
1.020-1.032 dilute 10 ml to 100 ml, take 10 ml 1.0
1.033-1.064 dilute 25 ml to 500 ml, take 10 ml 0.5
1.065-1.087 dilute 20 ml to 500 ml, take 10 ml 0.4
1.088-1.162 dilute 10 ml to 500 ml, take 10 ml 0.2
> 1.163 dilute 10 ml to 1,000 ml, take 10 ml 0.1
-
Reagents. The necessary reagents include silver nitrate, standard solution,
0.05N; potassium or sodium chromate, neutral 5% aqueous solution; and
nitric acid, 0.1N (nitrous free); and sodium bicarbonate.
Equipment. The necessary equipment includes a hotplate, a 10-ml micro-
buret, flasks, and pipets.
Procedure. After removal of interferences and selection of correct aliquot
size, dilute the sample to 20 ml or more, adjust the pH to 8.3 with sodium
bicarbonate or 0.1N nitric acid, add 1 ml of a 5% aqueous potassium chro-
mate solution, and titrate with an 0.05N silver nitrate solution until the red
endpoint just persists.
Calculation:
ml AgN03 x N x 35,500 = mg,l cl-
ml sample
The precision and accuracy of the method are about 1% and 2%, respec-
tively, of the amount present.
Bromide and iodide
Bromide and iodide are present in almost all petroleum-associated waters.
In the following procedure, iodide is selectively oxidized t'o iodate with
bromine water; excess bromine is reacted with sodium formate. The iodate
reacts with added iodide to produce iodine which is titrated with thiosulfate.
Hypochlorite is added to another sample to oxidize both bromide and iodide
to bromate and iodate, respectively. Excess hypochlorite is reacted with
sodium formate, and the bromate and iodate are reacted with iodide to
liberate iodine for titration with thiosulfate.