Page 67 - Geochemistry of Oil Field Waters
P. 67
FLAME SPECTROPHOTOMETRIC METHODS 55
26 - 1 'I I I I
-
~
2 4 0.01 mm rlit
22- - 1,620 volta to IT1 FW 6836
tOppri 02
x
-- 5psi C2H2
20-
0 18- 12.5 mm burner height
0
L -
-
-
-
-
-
5 10- -
-
L
-
-
-
-
mg Li/ml 50% n-PROPANOL
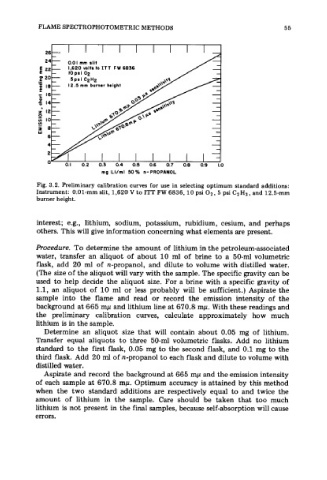
Fig. 3.2. Preliminary calibration curves for use in selecting optimum standard additions:
Instrument: 0.01-mm slit, 1,620 V to ITT FW 6836, 10 psi 02, 5 psi C2HZ, and 12.5-mm
burner height.
interest; e.g., lithium, sodium, potassium, rubidium, cesium, and perhaps
others. This will give information concerning what elements are present.
Procedure. To determine the amount of lithium in the petroleum-associated
water, transfer an aliquot of about 10 ml of brine to a 50-ml volumetric
flask, add 20 ml of n-propanol, and dilute to volume with distilled water.
(The size of the aliquot will vary with the sample. The specific gravity can be
used to help decide the aliquot size. For a brine with a specific gravity of
1.1, an aliquot of 10 ml or less probably will be sufficient.) Aspirate the
sample into the flame and read or record the emission intensity of the
background at 665 mp and lithium line at 670.8 mp. With these readings and
the preliminary calibration curves, calculate approximately how much
lithium is in the sample.
Determine an aliquot size that will contain about 0.05 mg of lithium.
Transfer equal aliquots to three 50-ml volumetric flasks. Add no lithium
standard to the first flask, 0.05 mg to the second flask, and 0.1 mg to the
third flask. Add 20 ml of n-propanol to each flask and dilute to volume with
distilled water.
Aspirate and record the background at 665 mp and the emission intensity
of each sample at 670.8 mp. Optimum accuracy is attained by this method
when the two standard additions are respectively equal to and twice the
amount of lithium in the sample. Care should be taken that too much
lithium is not present in the final samples, because self-absorption will cause
errors.