Page 49 - Glucose Monitoring Devices
P. 49
46 CHAPTER 3 Clinical evaluation of SMBG systems
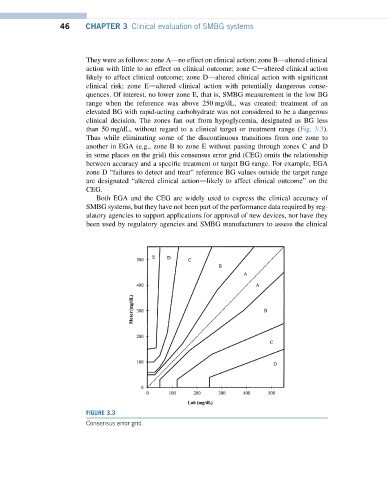
They were as follows: zone Adno effect on clinical action; zone Bdaltered clinical
action with little to no effect on clinical outcome; zone Cdaltered clinical action
likely to affect clinical outcome; zone Ddaltered clinical action with significant
clinical risk; zone Edaltered clinical action with potentially dangerous conse-
quences. Of interest, no lower zone E, that is, SMBG measurement in the low BG
range when the reference was above 250 mg/dL, was created: treatment of an
elevated BG with rapid-acting carbohydrate was not considered to be a dangerous
clinical decision. The zones fan out from hypoglycemia, designated as BG less
than 50 mg/dL, without regard to a clinical target or treatment range (Fig. 3.3).
Thus while eliminating some of the discontinuous transitions from one zone to
another in EGA (e.g., zone B to zone E without passing through zones C and D
in some places on the grid) this consensus error grid (CEG) omits the relationship
between accuracy and a specific treatment or target BG range. For example, EGA
zone D “failures to detect and treat” reference BG values outside the target range
are designated “altered clinical actiondlikely to affect clinical outcome” on the
CEG.
Both EGA and the CEG are widely used to express the clinical accuracy of
SMBG systems, but they have not been part of the performance data required by reg-
ulatory agencies to support applications for approval of new devices, nor have they
been used by regulatory agencies and SMBG manufacturers to assess the clinical
FIGURE 3.3
Consensus error grid.