Page 146 - Handbook of Thermal Analysis of Construction Materials
P. 146
130 Chapter 3 - Formation and Hydration
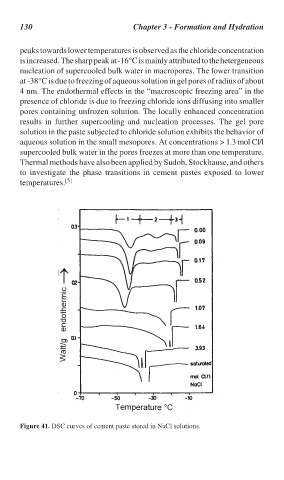
peaks towards lower temperatures is observed as the chloride concentration
is increased. The sharp peak at -16°C is mainly attributed to the hetergeneous
nucleation of supercooled bulk water in macropores. The lower transition
at -38°C is due to freezing of aqueous solution in gel pores of radius of about
4 nm. The endothermal effects in the “macroscopic freezing area” in the
presence of chloride is due to freezing chloride ions diffusing into smaller
pores containing unfrozen solution. The locally enhanced concentration
results in further supercooling and nucleation processes. The gel pore
solution in the paste subjected to chloride solution exhibits the behavior of
aqueous solution in the small mesopores. At concentrations > 1.3 mol Cl/l
supercooled bulk water in the pores freezes at more than one temperature.
Thermal methods have also been applied by Sudoh, Stockhause, and others
to investigate the phase transitions in cement pastes exposed to lower
temperatures. [5]
Figure 41. DSC curves of cement paste stored in NaCl solutions.