Page 148 - Handbook of Thermal Analysis of Construction Materials
P. 148
132 Chapter 3 - Formation and Hydration
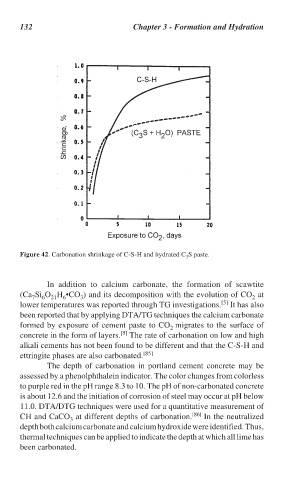
Figure 42. Carbonation shrinkage of C-S-H and hydrated C S paste.
3
In addition to calcium carbonate, the formation of scawtite
(Ca Si O H •CO ) and its decomposition with the evolution of CO at
7 6 21 6 3 2
[5]
lower temperatures was reported through TG investigations. It has also
been reported that by applying DTA/TG techniques the calcium carbonate
formed by exposure of cement paste to CO migrates to the surface of
2
[5]
concrete in the form of layers. The rate of carbonation on low and high
alkali cements has not been found to be different and that the C-S-H and
ettringite phases are also carbonated. [85]
The depth of carbonation in portland cement concrete may be
assessed by a phenolphthalein indicator. The color changes from colorless
to purple red in the pH range 8.3 to 10. The pH of non-carbonated concrete
is about 12.6 and the initiation of corrosion of steel may occur at pH below
11.0. DTA/DTG techniques were used for a quantitative measurement of
CH and CaCO at different depths of carbonation. [86] In the neutralized
3
depth both calcium carbonate and calcium hydroxide were identified. Thus,
thermal techniques can be applied to indicate the depth at which all lime has
been carbonated.