Page 212 - Handbook of Thermal Analysis of Construction Materials
P. 212
196 Chapter 5 - Accelerating Admixtures
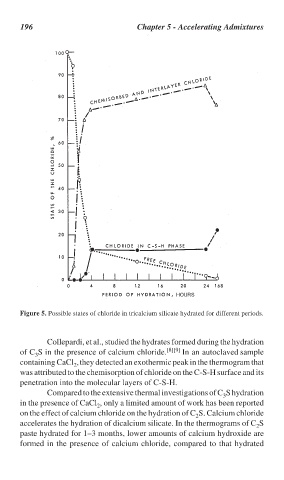
Figure 5. Possible states of chloride in tricalcium silicate hydrated for different periods.
Collepardi, et al., studied the hydrates formed during the hydration
of C S in the presence of calcium chloride. [8][9] In an autoclaved sample
3
containing CaCl ,they detected an exothermic peak in the thermogram that
2
was attributed to the chemisorption of chloride on the C-S-H surface and its
penetration into the molecular layers of C-S-H.
Compared to the extensive thermal investigations of C S hydration
3
in the presence of CaCl , only a limited amount of work has been reported
2
on the effect of calcium chloride on the hydration of C S. Calcium chloride
2
accelerates the hydration of dicalcium silicate. In the thermograms of C S
2
paste hydrated for 1–3 months, lower amounts of calcium hydroxide are
formed in the presence of calcium chloride, compared to that hydrated