Page 214 - Handbook of Thermal Analysis of Construction Materials
P. 214
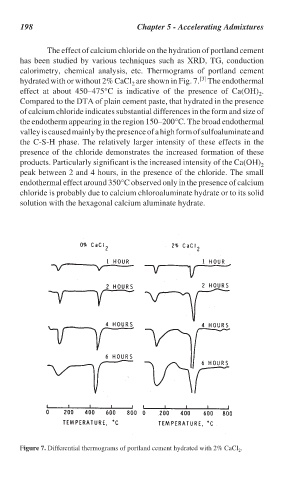
198 Chapter 5 - Accelerating Admixtures
The effect of calcium chloride on the hydration of portland cement
has been studied by various techniques such as XRD, TG, conduction
calorimetry, chemical analysis, etc. Thermograms of portland cement
[3]
hydrated with or without 2% CaCl are shown in Fig. 7. The endothermal
2
effect at about 450–475°C is indicative of the presence of Ca(OH) .
2
Compared to the DTA of plain cement paste, that hydrated in the presence
of calcium chloride indicates substantial differences in the form and size of
the endotherm appearing in the region 150–200°C. The broad endothermal
valley is caused mainly by the presence of a high form of sulfoaluminate and
the C-S-H phase. The relatively larger intensity of these effects in the
presence of the chloride demonstrates the increased formation of these
products. Particularly significant is the increased intensity of the Ca(OH)
2
peak between 2 and 4 hours, in the presence of the chloride. The small
endothermal effect around 350°C observed only in the presence of calcium
chloride is probably due to calcium chloroaluminate hydrate or to its solid
solution with the hexagonal calcium aluminate hydrate.
Figure 7. Differential thermograms of portland cement hydrated with 2% CaCl .
2