Page 256 - Handbook of Thermal Analysis of Construction Materials
P. 256
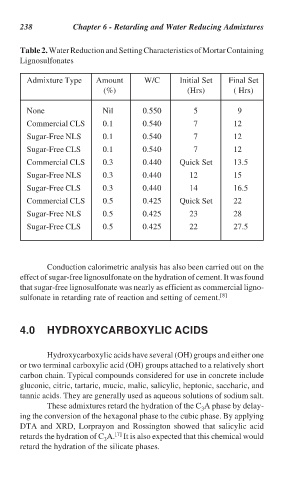
238 Chapter 6 - Retarding and Water Reducing Admixtures
Table 2. Water Reduction and Setting Characteristics of Mortar Containing
Lignosulfonates
Admixture Type Amount W/C Initial Set Final Set
(%) (Hrs) ( Hrs)
None Nil 0.550 5 9
Commercial CLS 0.1 0.540 7 12
Sugar-Free NLS 0.1 0.540 7 12
Sugar-Free CLS 0.1 0.540 7 12
Commercial CLS 0.3 0.440 Quick Set 13.5
Sugar-Free NLS 0.3 0.440 12 15
Sugar-Free CLS 0.3 0.440 14 16.5
Commercial CLS 0.5 0.425 Quick Set 22
Sugar-Free NLS 0.5 0.425 23 28
Sugar-Free CLS 0.5 0.425 22 27.5
Conduction calorimetric analysis has also been carried out on the
effect of sugar-free lignosulfonate on the hydration of cement. It was found
that sugar-free lignosulfonate was nearly as efficient as commercial ligno-
sulfonate in retarding rate of reaction and setting of cement. [8]
4.0 HYDROXYCARBOXYLIC ACIDS
Hydroxycarboxylic acids have several (OH) groups and either one
or two terminal carboxylic acid (OH) groups attached to a relatively short
carbon chain. Typical compounds considered for use in concrete include
gluconic, citric, tartaric, mucic, malic, salicylic, heptonic, saccharic, and
tannic acids. They are generally used as aqueous solutions of sodium salt.
These admixtures retard the hydration of the C A phase by delay-
3
ing the conversion of the hexagonal phase to the cubic phase. By applying
DTA and XRD, Lorprayon and Rossington showed that salicylic acid
[7]
retards the hydration of C A. It is also expected that this chemical would
3
retard the hydration of the silicate phases.