Page 327 - Handbook of Thermal Analysis of Construction Materials
P. 327
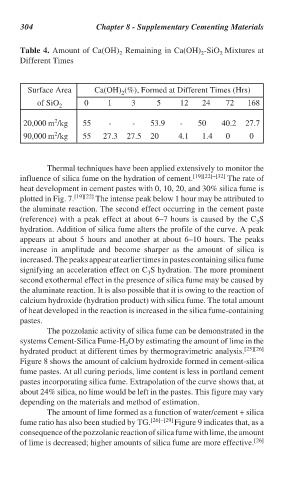
304 Chapter 8 - Supplementary Cementing Materials
Table 4. Amount of Ca(OH) Remaining in Ca(OH) -SiO Mixtures at
2
2
2
Different Times
Surface Area Ca(OH) (%), Formed at Different Times (Hrs)
2
of SiO 2 0 1 3 5 12 24 72 168
2
20,000 m /kg 55 - - 53.9 - 50 40.2 27.7
2
90,000 m /kg 55 27.3 27.5 20 4.1 1.4 0 0
Thermal techniques have been applied extensively to monitor the
influence of silica fume on the hydration of cement. [19][22]–[32] The rate of
heat development in cement pastes with 0, 10, 20, and 30% silica fume is
plotted in Fig. 7. [19][22] The intense peak below 1 hour may be attributed to
the aluminate reaction. The second effect occurring in the cement paste
(reference) with a peak effect at about 6–7 hours is caused by the C S
3
hydration. Addition of silica fume alters the profile of the curve. A peak
appears at about 5 hours and another at about 6–10 hours. The peaks
increase in amplitude and become sharper as the amount of silica is
increased. The peaks appear at earlier times in pastes containing silica fume
signifying an acceleration effect on C S hydration. The more prominent
3
second exothermal effect in the presence of silica fume may be caused by
the aluminate reaction. It is also possible that it is owing to the reaction of
calcium hydroxide (hydration product) with silica fume. The total amount
of heat developed in the reaction is increased in the silica fume-containing
pastes.
The pozzolanic activity of silica fume can be demonstrated in the
systems Cement-Silica Fume-H O by estimating the amount of lime in the
2
hydrated product at different times by thermogravimetric analysis. [25][26]
Figure 8 shows the amount of calcium hydroxide formed in cement-silica
fume pastes. At all curing periods, lime content is less in portland cement
pastes incorporating silica fume. Extrapolation of the curve shows that, at
about 24% silica, no lime would be left in the pastes. This figure may vary
depending on the materials and method of estimation.
The amount of lime formed as a function of water/cement + silica
fume ratio has also been studied by TG. [26]–[29] Figure 9 indicates that, as a
consequence of the pozzolanic reaction of silica fume with lime, the amount
of lime is decreased; higher amounts of silica fume are more effective. [26]