Page 329 - Handbook of Thermal Analysis of Construction Materials
P. 329
306 Chapter 8 - Supplementary Cementing Materials
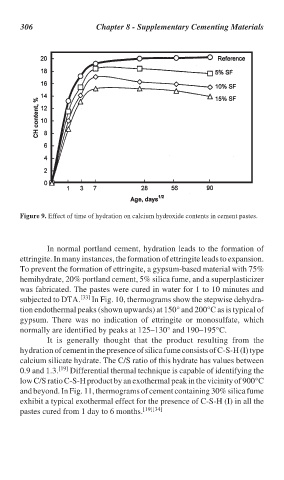
Figure 9. Effect of time of hydration on calcium hydroxide contents in cement pastes.
In normal portland cement, hydration leads to the formation of
ettringite. In many instances, the formation of ettringite leads to expansion.
To prevent the formation of ettringite, a gypsum-based material with 75%
hemihydrate, 20% portland cement, 5% silica fume, and a superplasticizer
was fabricated. The pastes were cured in water for 1 to 10 minutes and
subjected to DTA. [33] In Fig. 10, thermograms show the stepwise dehydra-
tion endothermal peaks (shown upwards) at 150° and 200°C as is typical of
gypsum. There was no indication of ettringite or monosulfate, which
normally are identified by peaks at 125–130° and 190–195°C.
It is generally thought that the product resulting from the
hydration of cement in the presence of silica fume consists of C-S-H (I) type
calcium silicate hydrate. The C/S ratio of this hydrate has values between
0.9 and 1.3. [19] Differential thermal technique is capable of identifying the
low C/S ratio C-S-H product by an exothermal peak in the vicinity of 900°C
and beyond. In Fig. 11, thermograms of cement containing 30% silica fume
exhibit a typical exothermal effect for the presence of C-S-H (I) in all the
pastes cured from 1 day to 6 months. [19][34]