Page 333 - Handbook of Thermal Analysis of Construction Materials
P. 333
310 Chapter 8 - Supplementary Cementing Materials
A granulated slag heated to a temperature of 800–900°C divitrifies
resulting in the appearance of one or more exothermal peaks in DTA. The
temperature, the number, and the intensity of the peaks differ from one slag
to the other. The area of the exothermic peak is indicative of the amount of
the glassy phase. The ground portland cement clinker does not exhibit any
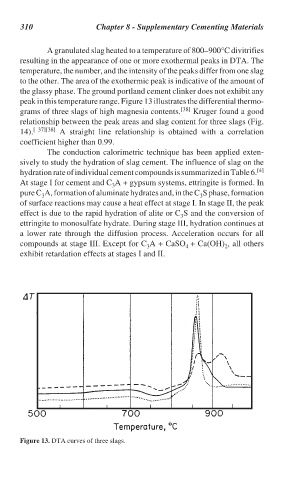
peak in this temperature range. Figure 13 illustrates the differential thermo-
grams of three slags of high magnesia contents. [38] Kruger found a good
relationship between the peak areas and slag content for three slags (Fig.
14). [ 37][38] A straight line relationship is obtained with a correlation
coefficient higher than 0.99.
The conduction calorimetric technique has been applied exten-
sively to study the hydration of slag cement. The influence of slag on the
hydration rate of individual cement compounds is summarized in Table 6. [4]
At stage I for cement and C A + gypsum systems, ettringite is formed. In
3
pure C A, formation of aluminate hydrates and, in the C S phase, formation
3
3
of surface reactions may cause a heat effect at stage I. In stage II, the peak
effect is due to the rapid hydration of alite or C S and the conversion of
3
ettringite to monosulfate hydrate. During stage III, hydration continues at
a lower rate through the diffusion process. Acceleration occurs for all
compounds at stage III. Except for C A + CaSO + Ca(OH) , all others
3 4 2
exhibit retardation effects at stages I and II.
Figure 13. DTA curves of three slags.