Page 469 - Handbook of Thermal Analysis of Construction Materials
P. 469
444 Chapter 10 - Non-Portland Rapid Setting Cements
7.0 HYDROXYAPATITE
Hydroxyapatites have been studied extensively due to their simi-
larity in composition to bone. Ten Huisen and Brown have described the
formation of hydroxyapatite by low temperature cementitious reactions
between various calcium phosphate precursors such as tetracalcium phos-
phate, Ca (PO ) O, and brushite (CaHPO •2H O). [29] The reaction involv-
4 2
4
4
2
ing the formation of stoichiometric hydroxyapatite (Ca/P = 1.67) (after
Brown and Chow [30] ) may be represented by the following reaction:
Eq. (7) 2CaHPO + 2Ca (PO ) O → Ca (PO ) (OH) 2
10
4
4
4 2
4 6
The formation of calcium-deficient hydroxyapatite (Ca/P = 1.50) can be
represented as follows:
Eq. (8) 3CaHPO + 1.5Ca (PO ) O → Ca (HPO )(PO ) OH
4 4 4 2 9 4 4 5
+ ½H O
2
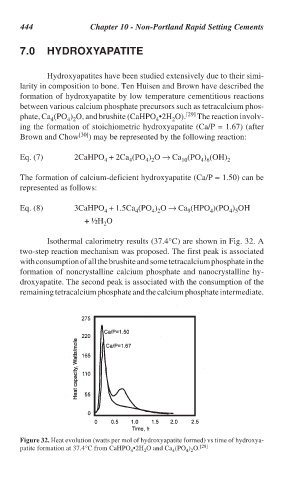
Isothermal calorimetry results (37.4°C) are shown in Fig. 32. A
two-step reaction mechanism was proposed. The first peak is associated
with consumption of all the brushite and some tetracalcium phosphate in the
formation of noncrystalline calcium phosphate and nanocrystalline hy-
droxyapatite. The second peak is associated with the consumption of the
remaining tetracalcium phosphate and the calcium phosphate intermediate.
Figure 32. Heat evolution (watts per mol of hydroxyapatite formed) vs time of hydroxya-
patite formation at 37.4°C from CaHPO •2H O and Ca (PO ) O. [28]
4 2 4 4 2