Page 470 - Handbook of Thermal Analysis of Construction Materials
P. 470
Section 7.0 - Hydroxyapatite 445
Martin and Brown investigated the hydration of tetracalcium
phosphate (C P) due to the fact that it is the only calcium phosphate more
4
basic than hydroxyapatite. [31] Its hydration was considered to be conceptu-
ally similar to that of the silicates. The reaction was considered to be
represented by the following equation:
Eq. (9) 3Ca (PO ) O + 3H O = Ca (PO ) (OH) + 2Ca(OH) 2
4 6
4 2
10
2
4
2
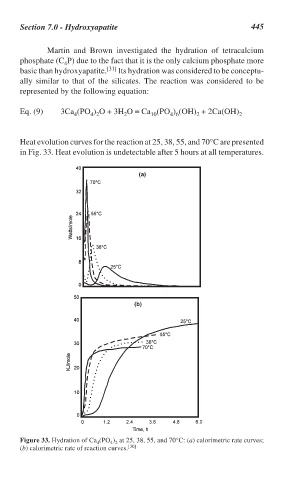
Heat evolution curves for the reaction at 25, 38, 55, and 70°C are presented
in Fig. 33. Heat evolution is undetectable after 5 hours at all temperatures.
Figure 33. Hydration of Ca (PO ) at 25, 38, 55, and 70°C: (a) calorimetric rate curves;
4
4 2
(b) calorimetric rate of reaction curves. [30]