Page 476 - Handbook of Thermal Analysis of Construction Materials
P. 476
Section 2.0 - DTA and DSC 451
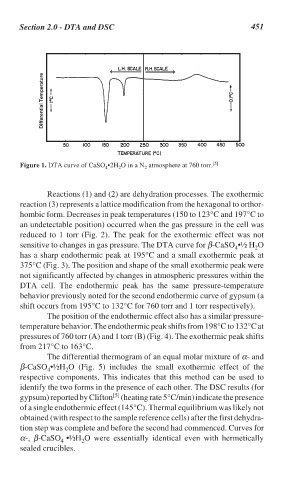
Figure 1. DTA curve of CaSO •2H O in a N atmosphere at 760 torr. [5]
2
2
4
Reactions (1) and (2) are dehydration processes. The exothermic
reaction (3) represents a lattice modification from the hexagonal to orthor-
hombic form. Decreases in peak temperatures (150 to 123°C and 197°C to
an undetectable position) occurred when the gas pressure in the cell was
reduced to 1 torr (Fig. 2). The peak for the exothermic effect was not
sensitive to changes in gas pressure. The DTA curve for β-CaSO •½ H O
4 2
has a sharp endothermic peak at 195°C and a small exothermic peak at
375°C (Fig. 3). The position and shape of the small exothermic peak were
not significantly affected by changes in atmospheric pressures within the
DTA cell. The endothermic peak has the same pressure-temperature
behavior previously noted for the second endothermic curve of gypsum (a
shift occurs from 195°C to 132°C for 760 torr and 1 torr respectively).
The position of the endothermic effect also has a similar pressure-
temperature behavior. The endothermic peak shifts from 198°C to 132°C at
pressures of 760 torr (A) and 1 torr (B) (Fig. 4). The exothermic peak shifts
from 217°C to 163°C.
The differential thermogram of an equal molar mixture of α- and
β-CaSO •½H O (Fig. 5) includes the small exothermic effect of the
4 2
respective components. This indicates that this method can be used to
identify the two forms in the presence of each other. The DSC results (for
[5]
gypsum) reported by Clifton (heating rate 5°C/min) indicate the presence
of a single endothermic effect (145°C). Thermal equilibrium was likely not
obtained (with respect to the sample reference cells) after the first dehydra-
tion step was complete and before the second had commenced. Curves for
α-, β-CaSO •½H O were essentially identical even with hermetically
4
2
sealed crucibles.