Page 107 - Handbook Of Multiphase Flow Assurance
P. 107
Hydrate of natural gas 103
TABLE 5.3 Number of hydrogen bonds in water at different temperatures
Temperature (°C) 0 25 50 100 150 200 250 300 350
% broken H-bonds in water 9 11 13.8 20 26 34 45 61 86.5
Average number of water molecules in 860 455 288 70 37 16 8 4 1–2
a cluster
After reference Makogon, Y.F., 1974. Gidraty Prirodnogo Gaza (Hydrates of Natural Gas, in Russian). Nedra, Moscow.
Vast majority of water molecules are hydrogen-bonded to their neighbors, with only a
small portion of bonds broken due to conformational defects, for example if two neighboring
water molecules point their hydrogen atoms in each other's direction. Broken bonds between
neighboring water molecules are eventually restored due to molecules' reorientations and
rearrangements. Some bonds are strained because of the differences in molecular positions.
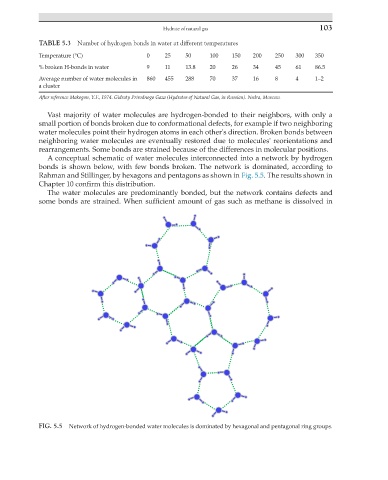
A conceptual schematic of water molecules interconnected into a network by hydrogen
bonds is shown below, with few bonds broken. The network is dominated, according to
Rahman and Stillinger, by hexagons and pentagons as shown in Fig. 5.5. The results shown in
Chapter 10 confirm this distribution.
The water molecules are predominantly bonded, but the network contains defects and
some bonds are strained. When sufficient amount of gas such as methane is dissolved in
FIG. 5.5 Network of hydrogen-bonded water molecules is dominated by hexagonal and pentagonal ring groups.