Page 108 - Handbook Of Multiphase Flow Assurance
P. 108
104 5. Flow restrictions and blockages in operations
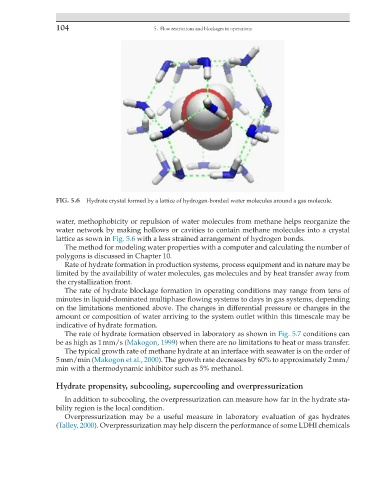
FIG. 5.6 Hydrate crystal formed by a lattice of hydrogen-bonded water molecules around a gas molecule.
water, methophobicity or repulsion of water molecules from methane helps reorganize the
water network by making hollows or cavities to contain methane molecules into a crystal
lattice as sown in Fig. 5.6 with a less strained arrangement of hydrogen bonds.
The method for modeling water properties with a computer and calculating the number of
polygons is discussed in Chapter 10.
Rate of hydrate formation in production systems, process equipment and in nature may be
limited by the availability of water molecules, gas molecules and by heat transfer away from
the crystallization front.
The rate of hydrate blockage formation in operating conditions may range from tens of
minutes in liquid-dominated multiphase flowing systems to days in gas systems, depending
on the limitations mentioned above. The changes in differential pressure or changes in the
amount or composition of water arriving to the system outlet within this timescale may be
indicative of hydrate formation.
The rate of hydrate formation observed in laboratory as shown in Fig. 5.7 conditions can
be as high as 1 mm/s (Makogon, 1999) when there are no limitations to heat or mass transfer.
The typical growth rate of methane hydrate at an interface with seawater is on the order of
5 mm/min (Makogon et al., 2000). The growth rate decreases by 60% to approximately 2 mm/
min with a thermodynamic inhibitor such as 5% methanol.
Hydrate propensity, subcooling, supercooling and overpressurization
In addition to subcooling, the overpressurization can measure how far in the hydrate sta-
bility region is the local condition.
Overpressurization may be a useful measure in laboratory evaluation of gas hydrates
(Talley, 2000). Overpressurization may help discern the performance of some LDHI chemicals