Page 116 - Handbook Of Multiphase Flow Assurance
P. 116
112 5. Flow restrictions and blockages in operations
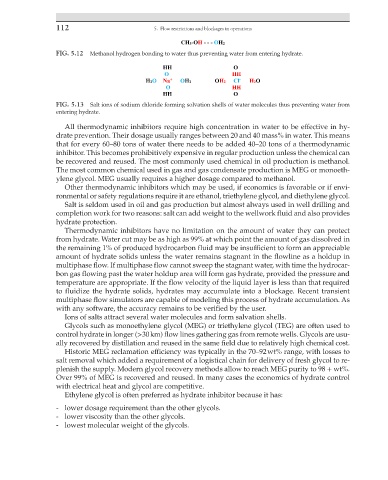
FIG. 5.12 Methanol hydrogen bonding to water thus preventing water from entering hydrate.
FIG. 5.13 Salt ions of sodium chloride forming solvation shells of water molecules thus preventing water from
entering hydrate.
All thermodynamic inhibitors require high concentration in water to be effective in hy-
drate prevention. Their dosage usually ranges between 20 and 40 mass% in water. This means
that for every 60–80 tons of water there needs to be added 40–20 tons of a thermodynamic
inhibitor. This becomes prohibitively expensive in regular production unless the chemical can
be recovered and reused. The most commonly used chemical in oil production is methanol.
The most common chemical used in gas and gas condensate production is MEG or monoeth-
ylene glycol. MEG usually requires a higher dosage compared to methanol.
Other thermodynamic inhibitors which may be used, if economics is favorable or if envi-
ronmental or safety regulations require it are ethanol, triethylene glycol, and diethylene glycol.
Salt is seldom used in oil and gas production but almost always used in well drilling and
completion work for two reasons: salt can add weight to the wellwork fluid and also provides
hydrate protection.
Thermodynamic inhibitors have no limitation on the amount of water they can protect
from hydrate. Water cut may be as high as 99% at which point the amount of gas dissolved in
the remaining 1% of produced hydrocarbon fluid may be insufficient to form an appreciable
amount of hydrate solids unless the water remains stagnant in the flowline as a holdup in
multiphase flow. If multiphase flow cannot sweep the stagnant water, with time the hydrocar-
bon gas flowing past the water holdup area will form gas hydrate, provided the pressure and
temperature are appropriate. If the flow velocity of the liquid layer is less than that required
to fluidize the hydrate solids, hydrates may accumulate into a blockage. Recent transient
multiphase flow simulators are capable of modeling this process of hydrate accumulation. As
with any software, the accuracy remains to be verified by the user.
Ions of salts attract several water molecules and form salvation shells.
Glycols such as monoethylene glycol (MEG) or triethylene glycol (TEG) are often used to
control hydrate in longer (>30 km) flow lines gathering gas from remote wells. Glycols are usu-
ally recovered by distillation and reused in the same field due to relatively high chemical cost.
Historic MEG reclamation efficiency was typically in the 70–92 wt% range, with losses to
salt removal which added a requirement of a logistical chain for delivery of fresh glycol to re-
plenish the supply. Modern glycol recovery methods allow to reach MEG purity to 98 + wt%.
Over 99% of MEG is recovered and reused. In many cases the economics of hydrate control
with electrical heat and glycol are competitive.
Ethylene glycol is often preferred as hydrate inhibitor because it has:
‐ lower dosage requirement than the other glycols.
‐ lower viscosity than the other glycols.
‐ lowest molecular weight of the glycols.