Page 166 - Handbook Of Multiphase Flow Assurance
P. 166
162 5. Flow restrictions and blockages in operations
crystallize first, at higher temperatures, and the short molecules crystallize last, at lower
temperatures.
Wax crystals typically contain molecules with carbon chains as short as 18 carbons, or
C 18 H 38 , octadecane. Crystals may contain n-paraffins as large as C 100 but these molecules ex-
ist in oils in very low quantities. More commonly, the heaviest n-paraffins present in a wax
deposit are in the C 40 –C 60 range. This range of the typical heaviest molecules determines
the typical wax appearance temperature (WAT), or the temperature at which the heaviest
n-paraffins begin to crystallize as wax at around 30 °C. There are oils with WAT as high as
50 °C, which contain high concentration of normal paraffins. Oils with high n-paraffin content
usually also exhibit high pour-point temperature and gelling issues, when the whole oil turns
into a solid-like non-Newtonian liquid.
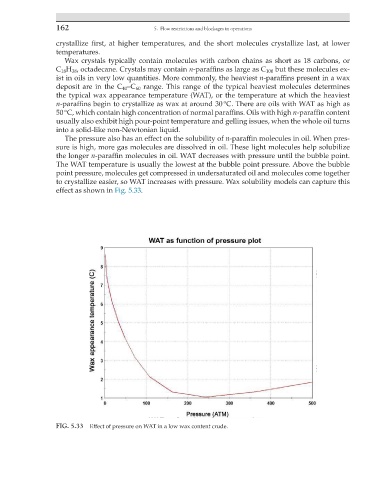
The pressure also has an effect on the solubility of n-paraffin molecules in oil. When pres-
sure is high, more gas molecules are dissolved in oil. These light molecules help solubilize
the longer n-paraffin molecules in oil. WAT decreases with pressure until the bubble point.
The WAT temperature is usually the lowest at the bubble point pressure. Above the bubble
point pressure, molecules get compressed in undersaturated oil and molecules come together
to crystallize easier, so WAT increases with pressure. Wax solubility models can capture this
effect as shown in Fig. 5.33.
FIG. 5.33 Effect of pressure on WAT in a low wax content crude.