Page 264 - Handbook Of Multiphase Flow Assurance
P. 264
Molecular modeling 263
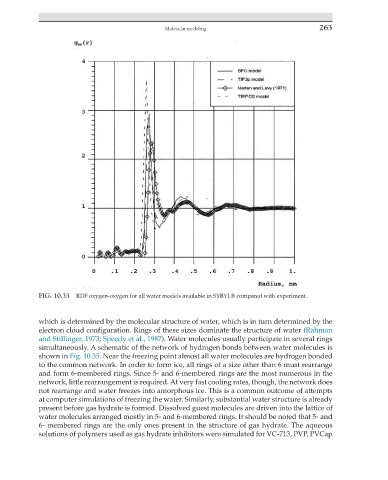
FIG. 10.33 RDF oxygen-oxygen for all water models available in SYBYL® compared with experiment.
which is determined by the molecular structure of water, which is in turn determined by the
electron cloud configuration. Rings of these sizes dominate the structure of water (Rahman
and Stillinger, 1973; Speedy et al., 1987). Water molecules usually participate in several rings
simultaneously. A schematic of the network of hydrogen bonds between water molecules is
shown in Fig. 10.35. Near the freezing point almost all water molecules are hydrogen bonded
to the common network. In order to form ice, all rings of a size other than 6 must rearrange
and form 6-membered rings. Since 5- and 6-membered rings are the most numerous in the
network, little rearrangement is required. At very fast cooling rates, though, the network does
not rearrange and water freezes into amorphous ice. This is a common outcome of attempts
at computer simulations of freezing the water. Similarly, substantial water structure is already
present before gas hydrate is formed. Dissolved guest molecules are driven into the lattice of
water molecules arranged mostly in 5- and 6-membered rings. It should be noted that 5- and
6- membered rings are the only ones present in the structure of gas hydrate. The aqueous
solutions of polymers used as gas hydrate inhibitors were simulated for VC-713, PVP, PVCap