Page 286 - Handbook Of Multiphase Flow Assurance
P. 286
Experimental and computer study of the effect of kinetic inhibitors on clathrate hydrates 285
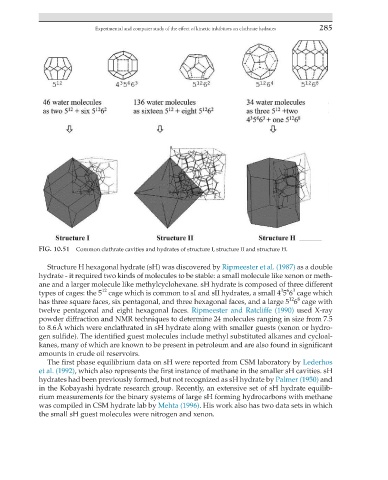
FIG. 10.51 Common clathrate cavities and hydrates of structure I, structure II and structure H.
Structure H hexagonal hydrate (sH) was discovered by Ripmeester et al. (1987) as a double
hydrate - it required two kinds of molecules to be stable: a small molecule like xenon or meth-
ane and a larger molecule like methylcyclohexane. sH hydrate is composed of three different
3 6 3
12
types of cages: the 5 cage which is common to sI and sII hydrates, a small 4 5 6 cage which
12 8
has three square faces, six pentagonal, and three hexagonal faces, and a large 5 6 cage with
twelve pentagonal and eight hexagonal faces. Ripmeester and Ratcliffe (1990) used X-ray
powder diffraction and NMR techniques to determine 24 molecules ranging in size from 7.5
to 8.6 Å which were enclathrated in sH hydrate along with smaller guests (xenon or hydro-
gen sulfide). The identified guest molecules include methyl substituted alkanes and cycloal-
kanes, many of which are known to be present in petroleum and are also found in significant
amounts in crude oil reservoirs.
The first phase equilibrium data on sH were reported from CSM laboratory by Lederhos
et al. (1992), which also represents the first instance of methane in the smaller sH cavities. sH
hydrates had been previously formed, but not recognized as sH hydrate by Palmer (1950) and
in the Kobayashi hydrate research group. Recently, an extensive set of sH hydrate equilib-
rium measurements for the binary systems of large sH forming hydrocarbons with methane
was compiled in CSM hydrate lab by Mehta (1996). His work also has two data sets in which
the small sH guest molecules were nitrogen and xenon.