Page 285 - Handbook Of Multiphase Flow Assurance
P. 285
284 10. Research methods in flow assurance
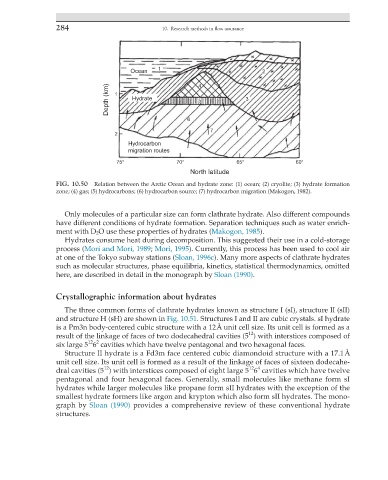
Ocean 1 2
Depth (km) 1 Hydrate 4 5 3
6
7
2
Hydrocarbon
migration routes
75° 70° 65° 60°
North latitude
FIG. 10.50 Relation between the Arctic Ocean and hydrate zone: (1) ocean; (2) cryolite; (3) hydrate formation
zone; (4) gas; (5) hydrocarbons; (6) hydrocarbon source; (7) hydrocarbon migration (Makogon, 1982).
Only molecules of a particular size can form clathrate hydrate. Also different compounds
have different conditions of hydrate formation. Separation techniques such as water enrich-
ment with D 2 O use these properties of hydrates (Makogon, 1985).
Hydrates consume heat during decomposition. This suggested their use in a cold-storage
process (Mori and Mori, 1989; Mori, 1995). Currently, this process has been used to cool air
at one of the Tokyo subway stations (Sloan, 1996c). Many more aspects of clathrate hydrates
such as molecular structures, phase equilibria, kinetics, statistical thermodynamics, omitted
here, are described in detail in the monograph by Sloan (1990).
Crystallographic information about hydrates
The three common forms of clathrate hydrates known as structure I (sI), structure II (sII)
and structure H (sH) are shown in Fig. 10.51. Structures I and II are cubic crystals. sI hydrate
is a Pm3n body-centered cubic structure with a 12 Å unit cell size. Its unit cell is formed as a
12
result of the linkage of faces of two dodecahedral cavities (5 ) with interstices composed of
12 2
six large 5 6 cavities which have twelve pentagonal and two hexagonal faces.
Structure II hydrate is a Fd3m face centered cubic diamondoid structure with a 17.1 Å
unit cell size. Its unit cell is formed as a result of the linkage of faces of sixteen dodecahe-
12 4
12
dral cavities (5 ) with interstices composed of eight large 5 6 cavities which have twelve
pentagonal and four hexagonal faces. Generally, small molecules like methane form sI
hydrates while larger molecules like propane form sII hydrates with the exception of the
smallest hydrate formers like argon and krypton which also form sII hydrates. The mono-
graph by Sloan (1990) provides a comprehensive review of these conventional hydrate
structures.