Page 287 - Handbook Of Multiphase Flow Assurance
P. 287
286 10. Research methods in flow assurance
Hydrate crystal growth
It is difficult to study the growth of the natural gas hydrate crystals, both because pressures
above atmospheric are required and because the hydrocarbon “guest” molecules are spar-
ingly soluble in water, so that crystallization occurs mainly at the gas-liquid interface (Long,
1994). It is more convenient to use tetrahydrofuran (THF) as a hydrate former because it is
completely miscible in water. Clathrate hydrates of THF melt at 4.38 °C at atmospheric pres-
sure (Erva, 1956). The THF hydrate is known to have a cubic structure (face-centered cubic,
diamondoid) (Palmer, 1950) and is classified as a structure II hydrate (Mak and McMullan,
1965). There are no published data about the shape of single THF hydrate crystals. A study of
shape of the negative crystals of THF hydrates (McLaurin and Whalley, 1988) showed that the
voids melted in a THF hydrate had an octahedral shape.
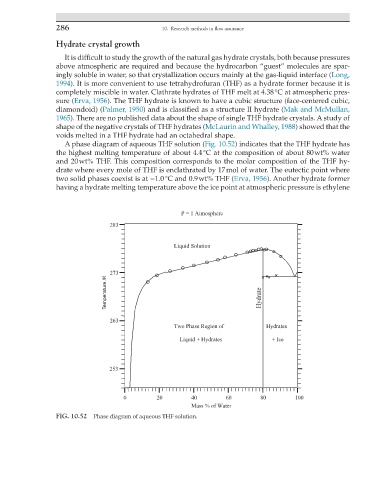
A phase diagram of aqueous THF solution (Fig. 10.52) indicates that the THF hydrate has
the highest melting temperature of about 4.4 °C at the composition of about 80 wt% water
and 20 wt% THF. This composition corresponds to the molar composition of the THF hy-
drate where every mole of THF is enclathrated by 17 mol of water. The eutectic point where
two solid phases coexist is at −1.0 °C and 0.9 wt% THF (Erva, 1956). Another hydrate former
having a hydrate melting temperature above the ice point at atmospheric pressure is ethylene
FIG. 10.52 Phase diagram of aqueous THF solution.