Page 280 - Handbook Of Multiphase Flow Assurance
P. 280
Molecular modeling 279
Normalized number
of hydrogen bonded rings
per molecule
0.3 SPC water T=203 (scaled 4C)
Normalized number of H bonded rings 0.15 PVP monomer in SPC water
0.25
0.2
PVcap monomer in SPC water
PVCA monomer in SPC water
0.1
0.05
0
1 2 3 4 5 6 7 8 9 10
Number of water molecules in H bonded ring
Number of water molecules in Hydrogen bonded ring.
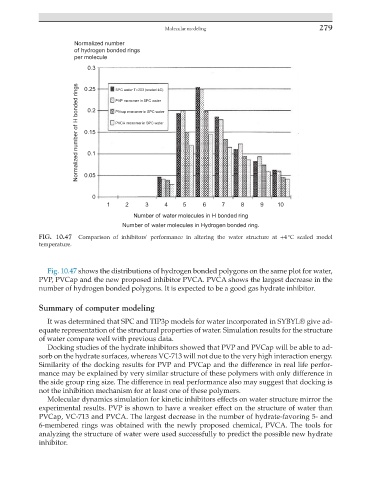
FIG. 10.47 Comparison of inhibitors' performance in altering the water structure at +4 °C scaled model
temperature.
Fig. 10.47 shows the distributions of hydrogen bonded polygons on the same plot for water,
PVP, PVCap and the new proposed inhibitor PVCA. PVCA shows the largest decrease in the
number of hydrogen bonded polygons. It is expected to be a good gas hydrate inhibitor.
Summary of computer modeling
It was determined that SPC and TIP3p models for water incorporated in SYBYL® give ad-
equate representation of the structural properties of water. Simulation results for the structure
of water compare well with previous data.
Docking studies of the hydrate inhibitors showed that PVP and PVCap will be able to ad-
sorb on the hydrate surfaces, whereas VC-713 will not due to the very high interaction energy.
Similarity of the docking results for PVP and PVCap and the difference in real life perfor-
mance may be explained by very similar structure of these polymers with only difference in
the side group ring size. The difference in real performance also may suggest that docking is
not the inhibition mechanism for at least one of these polymers.
Molecular dynamics simulation for kinetic inhibitors effects on water structure mirror the
experimental results. PVP is shown to have a weaker effect on the structure of water than
PVCap, VC-713 and PVCA. The largest decrease in the number of hydrate-favoring 5- and
6-membered rings was obtained with the newly proposed chemical, PVCA. The tools for
analyzing the structure of water were used successfully to predict the possible new hydrate
inhibitor.