Page 277 - Handbook Of Multiphase Flow Assurance
P. 277
276 10. Research methods in flow assurance
Normalized number
of hydrogen bonded rings
per molecule
0.25
0.2
0.15
0.1
0.05
0
1 2 3 4 5 6 7 8 9 10
Number of water molecules in hydrogen bonded ring.
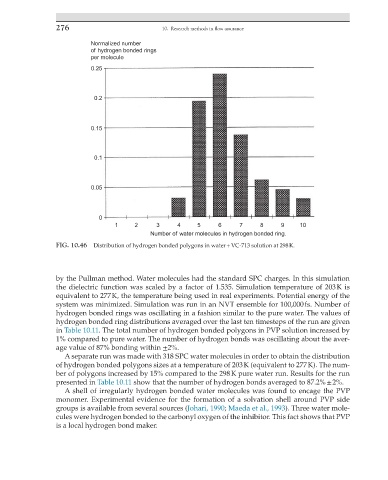
FIG. 10.46 Distribution of hydrogen bonded polygons in water + VC-713 solution at 298 K.
by the Pullman method. Water molecules had the standard SPC charges. In this simulation
the dielectric function was scaled by a factor of 1.535. Simulation temperature of 203 K is
equivalent to 277 K, the temperature being used in real experiments. Potential energy of the
system was minimized. Simulation was run in an NVT ensemble for 100,000 fs. Number of
hydrogen bonded rings was oscillating in a fashion similar to the pure water. The values of
hydrogen bonded ring distributions averaged over the last ten timesteps of the run are given
in Table 10.11. The total number of hydrogen bonded polygons in PVP solution increased by
1% compared to pure water. The number of hydrogen bonds was oscillating about the aver-
age value of 87% bonding within ±2%.
A separate run was made with 318 SPC water molecules in order to obtain the distribution
of hydrogen bonded polygons sizes at a temperature of 203 K (equivalent to 277 K). The num-
ber of polygons increased by 15% compared to the 298 K pure water run. Results for the run
presented in Table 10.11 show that the number of hydrogen bonds averaged to 87.2% ± 2%.
A shell of irregularly hydrogen bonded water molecules was found to encage the PVP
monomer. Experimental evidence for the formation of a solvation shell around PVP side
groups is available from several sources (Johari, 1990; Maeda et al., 1993). Three water mole-
cules were hydrogen bonded to the carbonyl oxygen of the inhibitor. This fact shows that PVP
is a local hydrogen bond maker.